Antioxidants


Please check back later for additional changes
Over the past decades, preventing oxygen pick-up during the brewing process and avoiding oxidation of the beer after fermentation has been adopted as efficient measures to resolve the beer aging issue. However, flavor stability remains hard to control despite extensive science and engineering research for years. The flavor stability of beer is increasingly found to be determined to a large extent by the endogenous antioxidant activity of beer itself (Zhao et al., 2010). Actually, beer itself with high endogenous antioxidant activity can prevent the generation of free radicals and these free radicals are formed only after the antioxidants in beer are deactivated. As the major endogenous antioxidants of beer, phenolic compounds are of particular interest to brewers because they play a key role in the brewing process by delaying, retarding, or preventing the oxidation processes.[1]
By definition, antioxidants are compounds which can delay or inhibit oxidative damage to substrates. (Poly-) phenols may act as antioxidants by different mechanisms: free radical inactivation by hydrogen atom transfer (HAT), or single electron transfer (SET) reactions, or by chelating prooxidant transition metal ions.[2]
Antioxidants are compounds that, in low concentration, can prevent biomolecules (proteins, nucleic acids, polyunsaturated lipids, sugars) from undergoing oxidative damage through free radical mediated reactions.[3] They can inhibit oxidizing chain reactions in several ways, including direct quenching of reactive oxygen species, inhibition of enzymes, and chelation of metal ions (Fe3+, Cu+).
The main antioxidants in beer are phenolic compounds, melanoidins, SO2, vitamins, etc.[4]
The AOX of beer not only contributes to the antioxidation and antiaging of beer itself in the process of fermentation and storage but also helps the drinker’s body to prevent oxidative stress. The composi tion and quantity of antioxidants in beer depend not only on the quality of raw materials but also on the technological process, so it is necessary to study the changes of antioxidants in barley and wort during the processes of germination, mashing, boiling, fermentation, etc.[4]
Antioxidants are compounds that have the ability to delay or prevent oxidation reactions (15) and are, therefore, thought to play a significant role in malting and brewing as inhibitors of oxidative damage. Antioxidants, including sulfites and ascorbic acid, can be added during brewing to successfully produce beers possessing a high level of antioxidant activity (39). However, in recent years, there has been a trend toward minimizing the use of added antioxidants because of consumer demand and stiffening regulations. As a result, attention needs to be paid to the protection and development of endogenous antioxidants during malting and brewing, e.g., phenolic compounds and Maillard reaction products, to produce beers with high levels of antioxidant activity and, therefore, increased resistance to lipid oxidation, an important contributor to beer staling.[5]
Although the total phenolic content in beer is lower than in white wines, the total anti-oxidant activity of beer is higher due to a higher content of some phenolic compounds (proanthocyanidin, epicatechin and ferulic acid).[6] Barley contains considerable amounts of phenolic antioxidants, mainly flavan-3-ol derivatives, phenolic acids and flavon glycosides. Among all classes of barley polyphenols, flavan-3-ol derivatives (anthocyanogens) are repeatedly mentioned in the literature due to their high biological activity and their presence in malts predominantly in free forms. On the other hand, these compounds are prone to transformation into dark-colored anthocyanidins at elevated temperatures, at decreased pH, and in the presence of oxygen.
Beer is the most important source of prenylflavonols of the diet. Prenylflavonols are metabolic compounds present in hops, responsible for various biological effects. Its composition depends on the variety of hops used, and the maturation and storage conditions. According to a study by Gorinstein et al., the concentration of procyanidins, epicatechins and ferulic acid is significantly higher in beer when compared to white wine, giving beer larger antioxidant capacity.[7]
Cu(II) is known to catalyze the oxidation of sulfite to sulfate by oxygen, yet metabisulfite had no effect on the 1-butanol. Clearly, any reactive oxygen species generated in the presence of sulfite is consumed at a much faster rate by unreacted sulfite than by 1-butanol. This property presumably contributes to sulfite's efficacy as a beer flavor stabilizer and suggested that sulfite might scavenge reactive oxygen species in the presence of prooxidants such as pyrogallol. A further model reaction study subsequently confirmed that an equimolar concentration of metabisulfite completely protected 1-butanol against oxidation in the presence of pyrogallol.[8] Furthermore, catechol and catechin were as efficient as metabisulfite in protecting 1-butanol against oxidation in the presence of pyrogallol. Thus, polyphenols such as catechin, which contain 1,2-dihydroxybenzene rings, not only are incapable of acting as prooxidants in coupled alcohol oxidations, but they actively protect alcohols from oxidation and therefore function as antioxidants. Monomeric and dimeric catechins can occur at levels of up to 5.5 and 4.0 mg/L, respectively, in beer (12,34) and should therefore help to stabilize beer flavor. Other dihydroxypolyphenols in beer, such as quercetin and leucocyanidin, are present in even higher concentrations (9) and should behave similarly.[8]
Melanoidins are occasionally invoked in beer-staling mechanisms. These poorly characterized, high molecular weight compounds are formed by reactions between sugars and amino acids and contain reducing groups called reductones. Most authors have found that melanoidins have antioxidant properties.[8] However,. ....The model melanoidin solution is known to contain predominantly unreacted sugars and amino acid, and subsequent control experiments showed that its ability to protect 1-butanol against oxidation in the presence of Cu(II) and pyrogallol was due to those unreacted starting materials. The latter are present at rather high concentrations and effectively scavenge the reactive oxygen species. Consequently, it is not possible to conclude from these results whether reductones have any prooxidant or antioxidant effect on the Cu(II)-catalyzed oxidation of alcohols. There seems to be a weak prooxidant effect with Fe(III).
A model reaction study confirmed that an equimolar concentration of metabisulfite completely protected 1-butanol against oxidation in the presence of pyrogallol (a pro-oxidant).[8]
Metal-chelating agents and ligands are known to alter the reduction potential and the catalytic efficiencies of copper and iron in reactions with molecular oxygen and hydrogen peroxide. Chelating agents such as EDTA are frequently used as quenchers of metal ion redox activity during biochemical research and as antioxidants or preservatives in various food systems. Indeed, Blockmans et al reported that beer treated with EDTA retained its freshness longer.[8] Transition metal ions are also known to bind amino acids, and it seemed probable that they, too, might affect the oxidation of alcohols and the resulting production of aldehydes in beer.
The Cu(II)-catalyzed oxidation of alcohols is inhibited by EDTA, lysine, metabisulfite, and 1,2-dihydroxypolyphenol species. Lysine and EDTA inhibit the formation of aldehydes in aged beer.[8]
The production of aldehydes in beer by added hydrogen peroxide is inhibited by EDTA, and the addition of lysine reportedly improves the flavor stability of beer.[8] Melanoidins bind Fe(II) and Cu(II) more strongly than does EDTA, and it has been suggested that this ability contributes to their antioxidant properties. Although not well documented, dark malts have been alluded to as producing more flavor-stable beers than pale malts. Perhaps this effect can be ascribed to the higher melanoidin content and hence higher metal-binding capacity of dark malts.
Higher levels of "anti-oxidant" substances in the beer retard deterioration processes. These agents, which originate in the grist, seem to work in different ways. They compete for oxygen by being oxidized themselves, or they inhibit enzymes catalysing oxidations, and/or they "scavenge" free radicals. Anti-oxidants include sulphite and bisulphite ions, polyphenols and reductones, which are ene-diol substances resembling ascorbic acid, which are formed during Maillard reactions. These compounds are found in dark malts, which have long been known to have flavour-stabilizing properties.[9]
Antioxidants have been shown in laboratory studies to increase beta-amylase activity and debranching enzyme activity.[10][11] However, the practical significance of this is unknown, and there have been no reports of the combination of sulfites and ascorbic acid increasing beer attenuation.[12]
The addition of these antioxidants to beer before packaging is likely to have a benefit in terms of flavor stability only if the beer is exposed to air during packaging. If in-pack oxygen is minimized, the addition of these antioxidants to the beer before packaging is unlikely to improve flavor stability. In addition, there is some evidence that both (+)–catechin and ferulic acid can contribute negative attributes to the beer. The presence of (+)–catechin in beer has been linked to colloidal instability, whereas the degradation product of ferulic acid (4-vinylguaiacol) can contribute strong flavor and aroma to the beer that could be considered detrimental.[13]
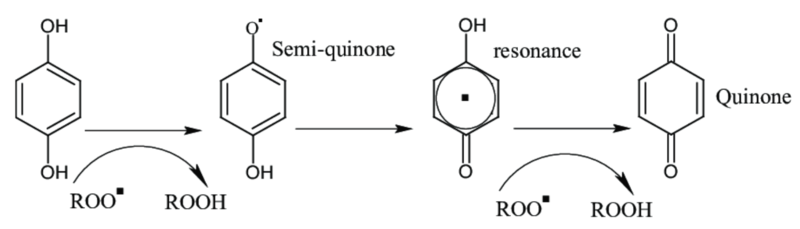
Ascorbic acid and sulfites are reducing agents that reduce quinones back to the original colorless phenols, or that react irreversibly with quinones to form stable colorless products.[14]
Ethylene diamine tetra acetic acid (EDTA) is a chelating agent that chelates the copper prosthetic group of PPO or reduces the level of copper available for incorporation into the holoenzyme (McEvily et al 1992). 4-Hexylresorcinol is a competitive inhibitor of PPO that has a molecular structure similar to that of the enzyme substrate and thus competes for the active site of the enzyme. 4-Hexylresorcinol has been used to prevent browning in shrimp and in fruits and vegetables (McEvily et al 1992; Vámos-Vigyázó 1995; Ashie et al 1996). Benzoyl peroxide is a bleaching agent that bleaches out pigments such as xanthophylls (Melland et al 1984).[14] 4-Hexylresorcinol (0.05 mg/g of barley flour) is a GRAS food additive, but is not approved for cereal products. Among chemical agents, the use of ascorbic acid resulted in significantly higher brightness of dough sheets at 24 hr for all four cultivars tested. Similar effects of ascorbic acid on retarding discoloration of oriental noodle doughs prepared with wheat flour were reported by Baik et al (1995). The second most effective antibrowning agent was 4- hexylresorcinol, a competitive inhibitor of PPO. Weemaes et al (1999) reported that 4-hexylresorcinol effectively inhibited mushroom PPO activity. However, 4-hexylresorcinol was neither a good inhibitor of grape PPO (Martinez and Whitaker 1995) nor effective in improving the brightness of wheat noodle dough.
In recent years, many endogenous antioxidants in beer, such as phenolic compounds, Maillard reaction products (MRPs), sulphite and chelating agents, had been reported responsible for flavour stability of beer (Vanderhaegen et al., 2006; Aron & Shellhammer, 2010). Among antioxidants mentioned above, phenolic compounds are of particular interest to brewers because they are described as antioxidants in vitro that possess antioxidant and antiradical properties as well as other biological effects (Rice-Evans et al., 1997; Gorinstein et al., 2007).[15]
The release of phenolic compounds and the formation of Maillard reaction products (MRPs) might be responsible for the increase in antioxidant activity during mashing. However, MRPs with antioxidant activity formed by the elevated temperatures during mashing might make major contribution to the increases in antioxidant activity at the stage of enzyme inactivation, because a rapid decrease in TPC at this stage was observed in this study. Indeed, Araki et al. (1999) observed increases in antioxidant activity levels during boiling for lager production and also attributed them to the formation of MRPs. Moreover, previous studies indicated that black beer with higher content of MRPs showed higher reducing power than lager beer (Lugasi & Ho´va´ri, 2003). Kilned and roasted malts also exhibited strong antioxidant properties because of the contributions of phenolic compounds and MRPs (Samaras et al., 2005). Therefore, phenolic compound and MRPs released from malt or formed during mashing process might make major contribution to antioxidant activity of mashes.[15]
Antioxidants are compounds that have a positive effect on human health. They are able to remove reactive oxygen and radicals from the organism and in this way prevent oxidative damage, such as ageing and degenerative diseases,cancer and cardiovascular disease.[16]
Beer is a homogeneous liquid wherein all components are evenly distributed throughout the medium. The efficiency of potential antioxidants therefore depends on how well these compounds compete with other components in beer as reaction partners with deleterious reactive species (radicals and reactive oxygen species). Polyphenols are present only in very low concentrations, which makes it kinetically impossible for polyphenols to efficiently trap highly reactive radicals and thus act as efficient antioxidants before these radicals react with other and much more abundant compounds in beer (especially ethanol).[17]
ascorbic acid and sulfites act in competition to polyphenol oxidation and are preferentiallv oxidized.[18] If very little ascorbic acid is used, it can be verified that the tannoids remain constant until the ascorbic acid is practically all oxidized. When the concentration of ascorbic acid tends toward zero, protection is no longer complete; and when all of the ascorbic acid has disappeared, the velocity of disappearance of the tannoids is slower than that of the control. Here there is found again a phenomenon similar to that cited in the subsection above; the oxidizing system appears to undergo destructive oxidation in the course of its functioning as an oxidation catalyst.
In complex food systems like beer, different classes of antioxidants (polyphenols, Maillard reaction products, SO2, antioxidant amino acids, hop resin substances, and so on) interact with each other both synergistically and antagonistically. Apart from that, the presence of metal ions and pH are important factors influencing total antioxidant capacity.[2]
Malted barley can have impact on beer stability due to the presence of pro-oxidant and antioxidant activities (1). Malt contains various compounds, originated from barley or formed during the malting process, which can play a significant role in malting and brewing through their antioxidant activities (1). Malt and barley contain, among others, phenolic compounds, phytic acid, ascorbic acid, melanoidins, and several enzymes which can be responsible for part of this total antioxidant power (2). Of these, melanoidins and polyphenols are the most significant sources of natural antioxidants, which can be originated from the malting process or already present in barley (3).[19]
The increase in the level of antioxidant activity observed during fermentation is likely because of the production of reduced nicotinamide adenine dinucleotide (NADH) by yeast, which is a by-product of the conversion of glucose to pyruvate during glycolysis. During fermentation, when sugars are abundant, their conversion to pyruvate/acetaldehyde may be more rapid than that of acetaldehyde to ethanol, leading to an excess of NADH in solution, and thus increased antioxidant activity levels in the beer. This is supported by Saha (35), who observed that NADH reaches a maximum after 120 hr of fermentation, indicating that levels would still be very high at the end of fermentation.[5]
Sulfite is also a natural product of yeast metabolism and can act as an antioxidant (4). Sulfite is first detected in beer after 15–20 hr of fermentation (24). Levels have been reported to reach a maximum after 100 hr and to remain constant after this time (13), suggesting that sulfites may also be playing a role in antioxidant activity.[5]
The antioxidant activity of malt and the corresponding wort is due not only to the phenolic compounds, but also to the products of the Maillard reaction.[20]
During fermentation the antioxidant activity decreased in some cases or showed no changes.[21]
Wort with dark specialty malts contains increased antioxidant activity.[22]
Antioxidants are important compounds that help us stay healthy, attenuating the oxidative stress which arises from overproduction of reactive oxygen or nitrogen species (ROS/RNS). The collective terms "reactive oxygen species (ROS) and reactive nitrogen species (RNS)" have been applied to a variety of free radicals such as superoxide, hydroxyl, peroxyl, nitric oxide, nitrogen dioxide radicals, as well as to non-radical reactive intermediates like hydrogen peroxide (H2O2) and peroxynitrite (ONOO−). These free radicals are currently produced under normal physiological conditions in our organism, but its generation is exacerbated under pathological conditions, and they play an important role in pathological processes and regulatory activities. Antioxidants can act in different ways, they can scavenge free radicals, inhibit prooxidative enzymes, and chelate metal ions, among others [2].[23]
The main antioxidant compounds in beer are phenolic compounds and melanoidins (formed throughout Maillard reaction). In addition, some antioxidant additives used in beer (i.e., vitamin C) may also contribute to its antioxidant capacity.[23]
As a result of the higher temperatures applied to produce special dark malts, higher levels of antioxidants (reductones and melanoidins) are formed from Maillard reactions. Consequently, beers with dark malts normally have a longer shelf life than pale beers.[23] In general, dark beers have higher antioxidant activity [39]. The fact that dark beers have a high antioxidant activity may be due to the use of special malts such as caramel malts or malts with different colorations. During the boiling process of these malts, different Maillard compounds are generated that have antioxidant activity [66]. By regions, Belgian beers tend to have greater antioxidant activity than Portuguese beers [103]. Asian beers present a lower FRAP value than English or German beers, because generally Asian beers are less bitter [104]. Bitter beers have a higher antioxidant activity, bitterness comes from hops and this is responsible for certain phenolic compounds such as procyanidins, epicatechin or ferulic acid being released during beer brewing, increasing the antioxidant activity [104]. The main mechanism by which hop-derived acids act as antioxidants is by iron chelation and by scavenging radicals [105].
On the basis of reaction mechanisms involved in the free radicals reduction processes, the methods to determine the antioxidant capacity are divided into two general groups: Methods based on the Single Electron Transfer (SET) and methods based on the Hydrogen Atom Transfer (HAT) [99]. The result is the same: the inactivation of free radicals, however, the kinetics and secondary reactions involved in the process are different.[23]
Single Electron transfer (SET): YXH + R• ⟶ YXH+• + R− Hydrogen atom transfer (HAT): YXH + R• ⟶ YX • + RH YXH: Antioxidant, R•: Free radical
In SET methods, an electron is provided by the antioxidant to the free radical and then the antioxidant becomes a radical cation, whereas in HAT methods the free radical removes one hydrogen atom from the antioxidant, and the antioxidant becomes a radical [61].[23] Phenolic compounds can undergo both HAT and SET, way depends mainly on the chemical structure of the phenolic compounds. Tocopherol, followed by hydroxytyrosol, gallic acid, caffeic acid, and epicatechin are the compounds most likely to go by HAT. Resveratrol and kaempferol are better suited for SET [61]. Several works carried out have shown that gallic acid and caffeic acid are the main phenols present in beer [47], so it can be assumed that HAT is the antioxidant mechanism that occurs mostly in beer.[23]
SO2 has a remarkably high affinity for oxygen, and the reaction speed is very fast. Oxygen can be removed in a very short time. SO 2 first reacts with oxygen molecules to form sulfates, thus preventing other sub stances in beer from reacting with oxygen to produce carbonyl com pounds, improving the antioxidation of beer. In addition, SO2 has the ability to scavenge free radicals during beer storage, which can prevent a chain reaction of free radicals and is conducive to the stability of beer flavor. Light and certain enzyme systems promote the production of reactive oxygen species. Particularly important are transition metal ions, such as iron and copper, which can effectively promote the formation of oxygen free radicals and multiple interconversions (Bamforth, 2001). In beer, sulfites are the only compounds that can delay the formation of oxygen free radicals, while phenolic compounds such as phenolic acid, catechin, epicatechin and proanthocyanidin dimer cause no delay in the formation of oxygen free radicals (Andersen, Outtrup, & Skibsted, 2000). Once the endogenous AOX of beer has decreased during storage, it is probably due to the obvious decrease in SO2 content, resulting in the enhancement of chain oxidation catalyzed by transition metal ions, especially iron ions (Guido, 2005; Karabín, Jelínek, Wietstock, & Dos tálek, 2014). Stepwise regression analysis has shown that the contribution of total SO2 content to the AOX of beer is 5% (Zhao et al., 2013). However, at present, SO2 and sulfites are considered to be allergens, and concerns about their safety as food additives are also increasing (Guido, 2016). Therefore, SO2 is generally used discreetly for anti oxidation and antiaging of beer products.[4]
Antioxidants in beer also include other compounds, such as vitamins B6, B12, E and C and selenium.[4] Selenium is part of the active site of glutathione peroxidase, both of which may contribute to the overall AOX in the body.
Antioxidants in beer are still being discovered. Certain new antiox idants have been isolated from beers recently, such as quercetin-3-O-(6′′ - malonyl)-glucoside, saponarin and hordatines A-C, which need to be further studied (Spreng & Hofmann, 2018). [4]
MRPs have been identified as the major contributors to the antioxidant activity of roasted malts (Coghe and others 2003, 2006; Samaras and others 2005), with a positive influence on the maintenance and development of malt reducing properties (Cechovsk ˇ a and others 2012).[24]
According to Goupy and others (1999), carotenoids (lutein and zeaxanthin) and tocopherols (α, β, and γ ) are also natural antioxidants of barley and malt.[24]
Some enzymes contained in barley or synthesized during germination can have antioxidant activity. Superoxide dismutase (SOD) catalyzes the dismutation of superoxide radical to hydrogen peroxide which is then decomposed into H2O and oxygen by means of catalase (CAT). By their sequential action, both enzymes act to maintain oxygen in the groundstate which is much less reactive than the superoxide and hydroperoxide. These enzymes are present in barley, and their activities increase not only during germination but also survive mild kilning, being destroyed at mashing temperatures above 65 °C (Bamforth 1991). Peroxidase (POD) is able to protect against oxidation by removing hydrogen peroxide. In this context it constitutes an endogenous primary antioxidant, but malt POD is also capable to oxidize endogenous barley phenolic compounds, such as ferulic acid, (+)-catechin and (–)-epicatechin (Boivin 2001). Residual enzyme activities in malt will depend on both the barley cultivar and the malting process.[24]
The determination of antioxidant properties of foods and biological systems is very difficult due to their complexity involving a variety of mechanisms such as: free radical chain breaking, oxygen scavenging, singlet oxygen quenching, metal chelation, and inhibition of oxidative enzymes.[24]
Hindered phenols are the most common antioxidant compounds to readily scavenge lipid peroxyl radicals by donating hydrogen atoms (Frankel and Meyer 2000). Metal chelators can be considered preventive antioxidants since metal-catalyzed initiation reactions and decomposition of lipid hydroperoxides can be inhibited due to the chelation of transition metal ions (Frankel 2005).[24]
There are many endogenous antioxidants such as polyphenols, Maillard reaction products, and sulfite present in beer. Among these antioxidants, polyphenols are of particular interest to brewers because they play a key role in the brewing process by delaying, retarding, or preventing oxidation processes (Lugasi and Hovari, 2003; Lu et al., 2007; Zhao et al., 2010).[25]
Only fresh or properly stored antioxidants (such as hop tannin extract) should be used for brewing purposes.[26]
barley and malt contains carotenoids, maily lutein and zeaxanthin. Barley and malt also contain tocopherols. d-Tocopherol and g-tocopherol are present in higher amounts than a-tocopherol.[27]
Addition of (+)- catechin and ferulic acid resulted in decreased carbonyl compounds formed during storage, in the presence of oxygen in the headspace, but both antioxidants did not result in a decrease of carbonyl compound formation in the case when the access of oxygen was limited. Addition of (+)-catechin and ferulic acid to beer before packaging can be applied to serve as an antioxidant31.[28]
The effect of O2-entrainment can be corrected by the addition of anti-oxidants, such as SO2 or gallotannins.[29]
Studies have shown that there may be another system within cereal grains whereby the reduction states of various proteins in the grain are controlled by the presence of a system composed of NADPH, thioredoxin, and the NADP-thioredoxin reductase (27, 28).[30]
The concentration of thiols has been shown to correlate to oxidative stability determined by electron paramagnetic resonance spectroscopy (7), and long-term stored beers that were depleted of sulphite but contained reduced protein thiols have been found to be surprisingly flavor stable (5). It is well known that thiols, such as the tripeptide glutathione (GSH), are highly efficient antioxidants in living cells and work by reacting with reactive oxygen species through both non-radical and radical reactions (8). Rogers and Clarke (4) suggested that thiols react as catalysts in the removal of H2O2 in beer, and recently it was established that thiols are also capable of reacting at very fast rates through a radical reaction with the 1-hydroxylethyl radical, which is known to be the main radical species formed during beer aging processes (9). Scavenging of the 1-hydroxylethyl radical prevents the formation of other reactive oxygen species that induce further oxidative damage. The rates of scavenging the 1-hydroxylethyl radical by thiols were found to be competitive with the degradation of hop bitter acids and therefore likely to protect against oxidation mediated by the 1-hydroxylethyl radical (9). Therefore, increasing the concentration of protein thiols in the beer could be a means to increase the oxidative stability of the beer.[31]
LTP1 isolated or purified from beer has been found to exhibit high antioxidative activity in two different antioxidant assays (5), as well as being a highly efficient scavenger towards the 1-hydroxylethyl radical (9). These results suggest that LTP1 is an important protein in relation to the hypothesis about protein thiols being antioxidants in beer. A number of trypsin/α-amylase inhibitors have also been identified in beer (12,15), and these proteins contain between six and 12 cysteine residues according to the NCBI protein database, so they could also contribute significantly to the thiol concentration in the beer.[31]
The antioxidants ascorbate and gallic acid displayed ROS scavenging activity and induced the resistance of yeast cells against a broad range of oxidants using this assay. Lipoic acid showed no direct scavenging activity but does provide resistance to yeast cells against a range of oxidants.[32]
The rate of radical formation (ESR rate) quantifies the rate of oxidation in the absence of efficient antioxidants and is usually correlated with the Fe and Cu contents, as well as ethanol concentration.[33] Color also seemed to be highly correlated with ESR rate and negatively correlated with lag phase. The PCA plot revealed a close correlation between lag phase and sulfite content, as shown in Figure 4, indicating that PC1 described antioxidant capacity. Interestingly, thiol content was also placed along the PC1 axis, showing a correlation between the concentration of thiol groups and the antioxidant capacity of a beer. This is consistent with the data provided in Table II, which shows that the least oxidatively stable beers with no lag phase during ESR measurements also contained smaller concentrations of thiols (13.6–29 µM) compared with the most oxidatively stable beers. However, beer 3 was the only beer with a thiol content less than 29 µM and a lag phase, which is likely explained by the presence of sulfite (4 ppm). This observation suggests that sulfite is the primary antioxidant in beer and that thiols may play a secondary role.[33]
the grouping of beers according to protein content did not correlate with the thiol concentration or oxidative stability of the beers and was probably a result of the use of different raw materials and brewing processes.[33]
Several authors have shown that the addition of reducing agents to a mash improves wort separation by breaking the interactions between proteins.[34]
A possible working mechanism for the ROS-scavenging ability of LTP1 or other proteins with free thiols: LTP thiol(s) is oxidised to the sulfenic acid by oxidants such as H2O2, which results in the destruction of a peroxide molecule in 1:1 stoichiometry. The free thiol can be recovered by two sequential reactions (reactions 2 and 3). The reaction 2 generates a disulfide (LTP-SSR) through reaction with a small molecule (HS-R) such as yeast thioredoxin. The reaction 3 uses sulfite or phenolic compounds to generate free thiol from the disulfide for the next round elimination of ROS.[35]
Oxidation is delayed if the operation is carried out in the presence of an excess of reducing agent such as an ascorbate or a sulfite.[18]
Addition of hop polyphenols to the brewing and sparging liquor resulted in a decrease in wort filtration time of approximately 15%. Similar positive effects of other polyphenols such as gallotannins on wort filterability were described earlier by Aerts et al (1). Addition of polyphenols to the brewing and sparging liquor may inhibit the oxidation of gel-forming proteins which normally facilitates coagulation and flocculation of proteins, thus resulting in accelerated wort filtration (1).[36]
anti-oxidative effects through iron complexation are seen with hop acids[37] Amino acids and melanoidins have been known to chelate copper ions [16], which is also the reason why the ‘hot break’ (which largely consists out of coagulated protein) is rich in metals [17]. Certain polyphenols, like prenylflavonoids and pro-anthocyanidins, should also act as anti-oxidants by similar metal-confining mechanisms, yet some debate still exists [18, 19].
DMSO, a natural component of all beers (1), is a "classic" hydroxyl scavenging agent used in experimental systems (36). Its significance as an antioxidant in beers has not been explored.[38]
Providing the beer with compounds, which compete with flavour active compounds to be oxidised by the oxidising agents present:[39]
- As stated earlier raw materials have a profound effect on the staling capacity of a beer. The use of dark malts and high hop grists give beer better keeping qualities.
- Naturally conditioned beer has yeast present in the final package and the yeast cells are able to scavenge residual oxygen picked up during filling.
- Sulphur dioxide is an anti-oxidant produced during fermentation. The levels of naturally occurring SO2 can be boosted during fermentation by deceasing yeast growth through:
- Lower fermentation temperatures
- Reduced wort aeration
- Reduced pitching rate
- Reduced original gravity
- Increased sulphate additions to the mash
- Producing bright worts
- Anti oxidants such as sulphur dioxide and ascorbic acid (or sodium ascorbate) can also be added to the beer, usually prior to packaging. It is found that the two antioxidants added together is the most effective method of use.
- Avoidance of metal ions, particularly iron and copper will reduce the rate of oxidation. This can be controlled through specifications on materials such as syrups and kieselguhr, and by diverting pre-coat liquors to drain to wash the filter bed out.
Recent investigations found that hop α-acids and β-acids, for example, can firmly bind to Fe ions, thereby diminishing radical reactions (45,46), but show only small chelating tendencies toward Zn ions (21). Therefore, in brewing practice, this feature from hop α-acids can be used to enhance the oxidative beer flavor stability (21).[40] However, certain polymeric phenols or phytic acids also are known to be good Fe chelators (2,37).
- Spreng, & Hofmann. (2018). Activity-guided identification of in vitro antioxidants in beer. Journal of Agricultural and Food Chemistry, 66, 720–731.
- http://www.themodernbrewhouse.com/brewing-methods/low-oxygen-brewing-malt-antioxidants/
- http://www.academia.edu/download/48805019/DPPH-original_LebensWissTechnol_1995-v28-p25.pdf
- https://pdfs.semanticscholar.org/3cc1/a7a3c97bce9130ef2184b346d9c35d38dedc.pdf
- Beer increases plasma antioxidant capacity in humans.
- http://www.themodernbrewhouse.com/wp-content/uploads/2016/11/ANTIOXINSBT.pdf Antioxin SBT
- http://www.themodernbrewhouse.com/wp-content/uploads/2016/11/ANTIOXINSB.pdf Antioxin SB (also repackaged as Antiox-c)
- http://www.themodernbrewhouse.com/wp-content/uploads/2016/11/presentation-philippe-cario.pdf Presentation on Antioxin SBT For Use in Belgian Beer
- http://www.themodernbrewhouse.com/wp-content/uploads/2016/11/Antioxidant-from-mashing-to-improve-flavor-stability-IBD-Ghana-March-320131.pdf Antioxin Testing on Flavor and Stability
- http://www.themodernbrewhouse.com/wp-content/uploads/2016/10/Declerck_2009_flavour-stability_beer.pdf A New Alternative to Increase The Flavor Stability of The Beer. Using Antioxin SBT
- http://www.themodernbrewhouse.com/wp-content/uploads/2016/12/Antioxidants-and-Flavor-Stability-Diss-German-Wurzbacher-2011.pdf Antioxidants-and-Flavor-Stability-Diss-German-Wurzbacher-2011.pdf
- http://www.themodernbrewhouse.com/wp-content/uploads/2016/12/Antioxidants-and-Flavor-Stability-Translated-Wurzbacher-2011.pdf Antioxidants-and-Flavor-Stability-Translated-Wurzbacher-2011.pdf- Translated to English by LOB
- Walters, M. T. Natural antioxidants and flavour stability. Ferment 10:111-119, 1997.
- McEvily, A. J., Iyengar, R., and Otwell, W. S. 1992. Inhibition of enzymatic browning in foods and beverages. Crit. Rev. Food Sci. Nutr. 32:253-273.
- Ashie, I. N., Simpson, B. K., and Smith, J. P. 1996. Mechanisms for controlling enzymatic reactions in foods. Crit. Rev. Food Sci. 36:1-30.
- Marianne N. Lund, Anna C. Krämer, and Mogens L. Andersen . Antioxidative Mechanisms of Sulfite and Protein-Derived Thiols during Early Stages of Metal Induced Oxidative Reactions in Beer. Journal of Agricultural and Food Chemistry 2015, 63 (37) , 8254-8261.
- Activity-Guided Identification of in Vitro Antioxidants in Beer
- Study of Antioxidant Properties of Agents from the Perspective of Their Action Mechanisms
- Phenols and Melanoidins as Natural Antioxidants in Beer. Structure, Reactivity and Antioxidant Activity
- Huang D, Ou B, Prior RL (2005) The chemistry behind antioxidant capacity assays. J Agric Food Chem 53(6):1841–1856
- Zhao, H. Endogenous Antioxidants and Antioxidant Activities of Beers. In Processing and Impact on Antioxidants in Beverages; Elsevier: Amsterdam, The Netherlands, 2014; pp. 15–24.
- Bamforth. (2002). Nutritional aspects of beer—a review. Nutrition Research, 22, 227–237.
- Dabina–Bicka, Karklina, Rakcejeva, Sniedzane, & Kviesis. (2010). The dynamics of vitamins c and e in barley products during malting. Research for Rural Development.
See also[edit]
References[edit]
- ↑ Zhao H. Chapter 64: Effects of processing stages on the profile of phenolic compounds in beer. In: Preedy V, ed. Processing and Impact on Active Components in Food. Academic Press; 2015:533-539.
- ↑ a b Wannenmacher J, Gastl M, Becker T. Phenolic substances in beer: Structural diversity, reactive potential and relevance for brewing process and beer quality. Compr Rev Food Sci Food Saf. 2018;17(4):953–988.
- ↑ Leopoldini M, Marino T, Russo N, Toscano M. Antioxidant properties of phenolic compounds: H-atom versus electron transfer mechanism. J Phys Chem A. 2004;108(22):4916–4922.
- ↑ a b c d e Yang D, Gao X. Research progress on the antioxidant biological activity of beer and strategy for applications. Trends Food Sci Technol. 2021;110:754-764.
- ↑ a b c Pascoe HM, Ames JM, Chandra S. Critical stages of the brewing process for changes in antioxidant activity and levels of phenolic compounds in ale. J Am Soc Brew Chem. 2003;61(4):203–209.
- ↑ Szwajgier D. Dry and wet milling of malt. A preliminary study comparing fermentable sugar, total protein, total phenolics and the ferulic acid content in non-hopped worts. J Inst Brew. 2011;117(4):569–577.
- ↑ Siqueira PB, Bolini H, Macedo GA. O processo de fabricação da cerveja e seus efeitos na presença de polifenóis. (The beer manufacturing process and its effects on the presence of polyphenols.) Alimentos e nutrição. 2008;19(4):491–498.
- ↑ a b c d e f g Irwin AJ, Barker RL, Pipasts P. The role of copper, oxygen, and polyphenols in beer flavor instability. J Am Soc Brew Chem. 1991;49(3):140–149.
- ↑ Briggs DE, Boulton CA, Brookes PA, Stevens R. Brewing Science and Practice. Woodhead Publishing Limited and CRC Press LLC; 2004.
- ↑ Evans DE, Wallace W, Lance RCM, MacLeod LC. Measurement of beta-amylase in malting barley (Hordeum vulgare L.). II. The effect of germination and kilning. J Cereal Sci. 1997;26(2):241–250.
- ↑ MacGregor AW, Bazin SL, Macri LJ, Babb JC. Modelling the contribution of alpha-amylase, beta-amylase and limit dextrinase to starch degradation during mashing. J Cereal Sci. 1999;29(2):161–169.
- ↑ Sulfites and attenuation. The Modern Brewhouse website. 2020. Accessed November 2020.
- ↑ Walters MT, Heasman AP, Hughes PS. Comparison of (+)–catechin and ferulic acid as natural antioxidants and their impact on beer flavor stability. Part 2: Extended storage trials. J Am Soc Brew Chem. 1997;55(3):91–98.
- ↑ a b Quinde-Axtell Z, Powers J. Baik BK. Retardation of discoloration in barley flour gel and dough. Cereal Chem. 2006;83(4):385–390.
- ↑ a b Zhao H, Zhao M. Effects of mashing on total phenolic contents and antioxidant activities of malts and worts. Int J Food Sci Technol. 2012;47(2):240-247.
- ↑ Jurková M, Horák T, Hašková D, Čulík J, Čejka P, Kellner V. Control of antioxidant beer activity by the mashing process. J Inst Brew. 2012;118(2):230-235.
- ↑ Andersen ML, Skibsted LH. Modification of the levels of polyphenols in wort and beer by addition of hexamethylenetetramine or sulfite during mashing. J Agric Food Chem. 2001;49(11):5232–5237.
- ↑ a b Chapon L, Chemardin M. The dissolving and oxidation of malt tannoids on mashing-in. Proceedings from the Annual meeting of American Society of Brewing Chemists. 1964;22(1):244–258.
- ↑ Guido LF, Curto AF, Boivin P, Benismail N, Gonçalves CR, Barros AA. Correlation of malt quality parameters and beer flavor stability: multivariate analysis. J Agric Food Chem. 2007;55(3):728–733.
- ↑ Shopska V, Denkova-Kostova R, Dzhivoderova-Zarcheva M, Teneva D, Denev P, Kostov G. Comparative study on phenolic content and antioxidant activity of different malt types. Antioxidants. 2021;10(7):1124.
- ↑ Koren D, Kun S, Vecseri BH, Kun-Farkas G. Study of antioxidant activity during the malting and brewing process. J Food Sci Technol. 2019;56(8):3801–3809.
- ↑ Gąsior J, Kawa-Rygielska J, Kucharska AZ. Carbohydrates profile, polyphenols content and antioxidative properties of beer worts produced with different dark malts varieties or roasted barley grains. Molecules. 2020;25(17):3882.
- ↑ a b c d e f Martinez-Gomez A, Caballero I, Blanco CA. Phenols and melanoidins as natural antioxidants in beer. Structure, reactivity and antioxidant activity. Biomolecules. 2020;10(3):400.
- ↑ a b c d e Carvalho DO, Gonçalves LM, Guido LF. Overall antioxidant properties of malt and how they are influenced by the individual constituents of barley and the malting process. Compr Rev Food Sci Food Saf. 2016;15(5):927–943.
- ↑ Ganbaatar C, Kubáň V, Kráčmar S, Valášek P, Fišera M, Hoza I. Liquid chromatographic determination of polyphenenols in Czech beers during brewing proces. Potravinárstvo. 2015;9(1):24–30.
- ↑ Karabín M, Hanko V, Nešpor J, Jelínek L, Dostálek P. Hop tannin extract: a promising tool for acceleration of lautering. J Inst Brew. 2018;124(4):374–380.
- ↑ Goupy P, Hugues M, Boivin P, Amiot MJ. Antioxidant composition and activity of barley (Hordeum vulgare) and malt extracts and of isolated phenolic compounds. J Sci Food Agric. 1999;79(12):1625–1634.
- ↑ Szwajgier D, Pielecki J, Targoński Z. The release of ferulic acid and feruloylated oligosaccharides during wort and beer production. J Inst Brew. 2005;111(4):372–379.
- ↑ De Rouck G, Jaskula-Goiris B, De Causmaecker B, et al. The impact of wort production on the flavour quality and stability of pale lager beer. BrewingScience. 2013;66(1/2):1–11.
- ↑ Perrocheau L, Bakan B, Boivin P, Marion D. Stability of barley and malt lipid transfer protein 1 (LTP1) toward heating and reducing agents: relationships with the brewing process. J Agric Food Chem. 2006;54(8):3108−3113.
- ↑ a b Lund MN, Lametsch R, Sørensen MB. Increased protein–thiol solubilization in sweet wort by addition of proteases during mashing. J Inst Brew. 2014;120(4):467–473
- ↑ Wu MJ, Rogers PJ, Clarke FM. 125th anniversary review: The role of proteins in beer redox stability. J Inst Brew. 2012;118(1):1–11.
- ↑ a b c Lund MN, Andersen ML. Detection of Thiol Groups in Beer and Their Correlation with Oxidative Stability. J Am Soc Brew Chem. 2011;69(3):163–169.
- ↑ Muller R. Use of 5,5’-dithiobis (2-nitrobenzoic acid) as a measure of oxidation during mashing. J Am Soc Brew Chem. 1995;53(2):53–58.
- ↑ Wu MJ, Clarke FM, Rogers PJ, et al. Identification of a protein with antioxidant activity that is important for the protection against beer ageing. Int J Mol Sci. 2011;12(9):6089–6103.
- ↑ Jaskula-Goiris B, Goiris K, Syryn E, et al. The use of hop polyphenols during brewing to improve flavor quality and stability of pilsner beer. J Am Soc Brew Chem. 2014;72(3):175–183.
- ↑ Mertens T, Kunz T, Methner FJ. Assessment of chelators in wort and beer model solutions. BrewingScience. 2020;73(May/June):58–67.
- ↑ Bamforth CW, Muller RE, Walker MD. Oxygen and oxygen radicals in malting and brewing: a review. J Am Soc Brew Chem. 1993;51(3):79–88.
- ↑ O'Rourke T. The role of oxygen in brewing. Brewer International. 2002;2(3):45–47.
- ↑ Wietstock PC, Kunz T, Waterkamp H, Methner FJ. Uptake and release of Ca, Cu, Fe, Mg, and Zn during beer production. J Am Soc Brew Chem. 2015;73(2):179–184.