Yeast


Please check back later for additional changes
The earliest written account of brewing dates from Mesopotamian times. However, our understanding of the connection with yeast is relatively recent, starting with Leeuwenhoek’s microscope observations in the 17th century followed by the work of Lavoisier, Gay-Lussac, Schwann and others during the 18th and 19th centuries. It was not until the late 19th century that Pasteur demonstrated that fermented beverages result from the action of living yeast’s transformation of glucose (and other sugars) into ethanol. Since then, our knowledge has expanded exponentially, particularly with the development of molecular biology techniques.[1]
"Fermentation is life without oxygen." –Louis Pasteur, 1876
The conversion of the fermentable carbohydrates (sugars) into ethanol and carbon dioxide gas is achieved by pitching yeast. However, other by-yeast metabolism products are also excreted into the fermenting wort and can affect the organoleptic properties (i.e., taste, color, odor and feel) of the beer. These by-products include esters, aldehydes, vicinal diketones, higher alcohols and acids, as well as sulfur compounds.[2]
In beer, glucose, fructose, maltose, and maltotriose are all consumed simultaneously. The glucose, fructose and sucrose are depleted first because they are present in lower amounts. The rate of maltotriose consumption is slowest, and so it is the last sugar to be depleted.[3]
Boiled wort ferments more quickly than raw wort.[3] Why?
Maltose-rich worts ferment more quickly and hold the yeast in suspension longer.[4] Higher fermentability increases fermentation speed, alcohol content, and ester formation.[4]
High concentrations of glucose inhibit maltose and maltotriose consumption in some yeast strains.[5][6]
Average dextrin size in wort is reduced during fermentation. This indicates that yeast can also utilize dextrin molecules in addition to fermentable sugars. Yeast accomplish this by releasing amylolytic enzymes to degrade water soluble starch molecules.[7]
Kerr et al. (2018), used SWATH-MS to understand the causes of the flocculation behavior of yeast and to identify differences between brewing strains. Flocculation is important at the end of fermentation, in which yeast cells adhere together to form large flocs. This phenotype is highly desired after fermentation, as it allows easy removal of yeast cells from the beer (Govender et al., 2011; Verstrepen et al., 2003). Differences in Flo10 and Flo1/5 proteins responsible for flocculation behaviors were found in the cell walls of industrially relevant yeast (Kerr et al., 2018). This study also identified YIQ9, a homolog to Cfg1 (Carlsbergensis foaming gene) (Blasco et al., 2012; Kerr et al., 2018), in brewing yeasts, which was absent in the laboratory strain BY4743. Further, large differences were found between strains at the level of the global whole cell proteome (Kerr et al., 2018).[8]
Protein thiols are effective against inhibition of yeast growth in the presence of reactive oxygen species.[9][10] Yeast thioredoxin (TRX) is another thiol-rich candidate ripe for consideration as a functional element of anti-ROS cascades. At one time we anticipated that TRX, which is secreted by yeast during fermentation, could be linked to beer stability. That was in fact the start of our move into beer proteomics. Yeast cells protect themselves against oxidative stress with interlinking processes that can transfer electrons within cells to where they are needed to quench reactive oxygen species. More contentiously, they can transfer reducing equivalents to outside the cell, where presumably they may carry out different or perhaps the same defence strategies. The trouble with this view, at least on face value, is that, outside the cell, the rest of the machinery for recycling oxidized TRX is just not present. Yet it might be possible — if yeast happens to secrete small thiols like cysteine as some mammalian cells do — this might be how LTP is reduced during fermentation after probably being oxidized in the wort and in the kettle. Taking together the data of beer proteomics and thiol proteins, we propose that there could be a thiol-based cycle operating in beer that involves oxidized thiols and reversible reduction after peroxide destruction using sulfite or some reductant molecules.[10]
- "The impact of the physiological condition of the pitching yeast on beer flavour stability: an industrial approach"
- "Sugar utilization by yeast during fermentation."
- https://www.sciencedirect.com/science/article/pii/B9780127999548000046
While it is clear that the introduction of oxygen into wort can have a negative impact on quality, it is intentionally added during wort cooling. Yeast requires oxygen to synthesize UFAs and sterols, which are critical cell membrane components that impact membrane fluidity. Depraetere et al. showed that under normal conditions, oxygenation of cold wort (8 ppm O 2 ) had no significant impact on the level of aging staling indicators for ale or lager beers over a 12 month ambient shelf-life test (14). At this stage of the process, oxidation of wort compounds appears to play little to no part compared with the change of aroma and flavor from yeast metabolism. It is important to note that the wort oxygenation rate can impact the levels of sulfites produced by lager yeast; increased oxygenation can cause a reduction in sulfite levels, leading to a loss of scavenging activity for carbonyl compounds, such as E2N (22).[11]
- Depraetere, S., et al. 2008. The influence of wort aeration and yeast preoxygenation on beer staling processes. Food Chem. 107(1):242-249.
The ventilation of wort before fermentation is a necessary step to ensure the full growth and better fermentation of yeast (Depraetere, De Schutter, Williams, & Delvaux, 2008). With further increase of dissolved oxygen in wort, the content of SO2 decreases obviously, which indicates that too much dissolved oxygen in wort inhibits the production of SO2 (Dufour, 1991). That is, controlling dissolved oxygen can improve generation of SO2 and antioxidative activity of beer. The pitching rate of yeast was found to be able to control the fermentation time and the peak number of yeast cells and also had an effect on the composition and flavors of beer (Verbelen, Saerens, Thevelein, & Delvaux, 2009). Low pitching rate affects the multiplication of yeast during the main fermentation stage, results in a smaller reduction in apparent extract and retards fermen tation. In contrast, higher pitching rate leads to vigorous growth of yeast and rapid decline of apparent extract during the main fermentation stage. An increase in pitching rate leads to a decrease in the quantity of SO2 produced by fermentation. This may be explained in that when the pitching rate is high, the yeast metabolism is exuberant, so that more sulfites are metabolized by the yeast to synthesize the amino acids needed for its own metabolism, resulting in less SO2 (Zhou, 2010). Therefore, the appropriate pitching rate is crucial for generating SO2 during beer fermentation (Zhao, 2012).[12]
Higher wort gravity leads to greater SO2 production during fermentation (Zhou, 2010). Higher wort gravity increases the osmotic pressure of yeast cells and changes the metabolic pathway of yeast utilizing glucose, resulting in the production of more pyruvate, acetaldehyde, ethanol, etc., from glucose through glycolysis. Pyruvate and acetaldehyde are easily combined with sulfite, and the adduct products of sulfite and carbonyl compounds are secreted into the wort through cell membranes, increasing the content of SO2 in the wort (Gyllang, Winge, & Korch, 1989). However, when the wort gravity exceeds 12 ◦ P, the AOX of beer does not increase significantly as the wort gravity in creases, indicating that the antioxidant power of wort is affected not only by metabolic pathways but also by the reduction ability of yeast. In high gravity brewing, due to the inhibition of high osmotic pressure and high ethanol concentration, the metabolic reduction ability of yeast cells decreases, result in a sluggish generation of SO2 in wort, and the wort cannot reach the proper antioxidant level (Li, Sun, Zhao, & Zhao, 2012).[12]
Temperature is an important external factor affecting the participa tion of yeast in biochemical reactions, directly influencing the compo sition and content of metabolites including antioxidants in beer (Yu, Chen, & Wang, 2006). The effect of the main fermentation temperature on the TBA value is significant, and a lower main fermentation temperature leads to a lower TBA value of the wort. This may be because higher main fermentation temperature promotes the metabolism of yeast and produces more higher alcohols. In the process of yeast fermentation, pathways of amino acid degradation and the synthesis and metabolism of carbohydrates form higher alcohols, which are easily oxidized by melanoidin catalysis, resulting in the formation of aldehyde carbonyl compounds and thus higher TBA value (Zhao, 2012). Theoretically, higher temperature leads to stronger reproduction and metabolism of yeast, stronger reduction of aging substances and higher AOX of green beer. However, this is not the case. The DPPH radical scavenging activity, oxygen free radical absorption capacity and reducing power of beer have been found to decrease with increasing fermentation temperature (Li, SunZhao, & Zhao, 2012). This may be because the fermentation temperature affects the proliferation and metabolic rate of yeast, as well as the AOX of green beer. Higher fermentation temperature leads to shortened fermentation period, lower concentrations of reduction agents and insufficient reduction of aging precursors, while low temperature prolongs fermentation and produces more reduction agents, so that the AOX of the beer is higher. In addition, lower temperature is also good for keeping SO2 in wort, improving the antioxidant capacity of wort. Hence, low-temperature fermentation is more conducive to the maintenance of higher AOX in beer.[12]
The beer filtration process reduces the contents of antioxidant phenolic compounds and melanoidins and the AOX of wort. During the cooling stage, the spontaneous adsorption of phenolic compounds and melanoidins on wort dregs and the polymerization and precipitation of catechins and epicatechins lead to the decrease of TPC in beer (Ruiz- Ruiz, Del Carmen Esapadas Aldana, Cruz, & Segura-Campos, 2020). With the increase of diatomite consumption, a large concentration of iron ions is introduced, which decreases the DPPH scavenging rate, because transition ions such as iron and copper play an important cat alytic role in the Fenton reaction, producing hydroxyl free radicals with high activity and reducing the oxidation resistance of beer (Jurková et al., 2012; Pascoe, Ames, & Chandra, 2003). The addition of tannins has an obvious effect on the rate of scavenging of DPPH free radicals, indicating that the addition of tannins will help to chelate iron ions and reduce the effect of iron ions in diatomite on beer. The reducing power of beer can be improved by maintaining pH within the range 4.3–4.4 (Han, 2016). After cooling and filtration, 6% of selenium is lost from the level in raw materials, and the total loss of selenium over the whole process of beer fermentation is 94% (Rodrigo et al., 2015). It can be seen that the percentage selenium loss is quite high, which deserves attention.[12]
Pressure fermentation suppresses ester production.[13]
SafaleTM S-04 is a maltotriose negative yeast, thus sugars remain into the beer contributing to sweet character to aroma and taste.[14]
Yeast is regarded as the best "antioxidant" for brewing due to its strong ability to absorb dissolved oxygen.[15]
Reducing Activity of Yeast. It is generally accepted that yeast metabolism can reduce aldehydes in the wort to their corresponding alcohols. The system responsible for this reduction has been found to be very complex and heterogeneous.149,154 Some aldehyde reductases regenerate NAD(P)+ from NAD(P)H and, therefore, maintain a suitable redox balance within the cell.154,193 Spiking of aldehydes to wort with subsequent laboratory-scale fermentation results in a lack of measurable aldehyde levels directly after fermentation and yeast removal. Moreover, the malt-like aroma disappears completely by this fermentation step. On the other hand, the corresponding alcohols and acetate esters showed to be present.153,165,193 Collin et al.155 suggested that the limiting step of carbonyl reduction is the uptake rate by the yeast, but this was countered by the findings of Debourg et al.,149 who worked with permeabilized yeast cells. Linear saturated aldehydes appear to be reduced more rapidly with increasing carbon number, and their reduction rate is higher than their corresponding branched or unsaturated aldehydes.149 Furfural and (E)-2-nonenal are reduced early in the fermentation process.63,115,194 Vesely et al.195 observed a clear decrease in, among others, 2-methylpropanal, 2-methylbutanal, 3-methylbutanal, furfural, and methional concentrations, at both 10 and 15 °C fermentations. Although the reduction rates were slightly higher at 15 °C, the resulting aldehyde concentrations were lower at 10 °C. Perpete et al. ̀ 182,193 reported an initially fast reduction of Strecker aldehydes in cold contact fermentation, which slowed and resulted after a few hours in a constant concentration. This end concentration is aldehyde-dependent, but can reach up to 40% of the initial concentration. Higher fermentation temperatures led to lower, but nonzero, end concentrations. Neither higher pitching rates nor different yeast strains or even a second pitching with fresh yeast affected the concentration of aldehydes at the end of fermentation. Similar results were obtained with laboratory-scale and industrial fermentation trials. This points to the interactions of the aldehydes with wort components rendering them nonreducible by the yeast, for example, imine formation and bisulfite adduct formation, but also, for example, weak binding to flavonoids at fermentation temperatures.153,182,193 As the free aldehydes are reduced by the yeast, the equilibrium between free and bound aldehydes restores the free form, yet this seems insufficient for complete aldehyde reduction.149 Aldehyde reduction by the yeast starts very early in the fermentation process, whereas sulfite production occurs at a later time.75 The protective effect of sulfite binding is, therefore, thought to be of rather limited importance.182 Yeast also reduces α-dicarbonyls, the intermediates of the Maillard reaction pathway and part of the Strecker degradation pathway. Addition of an isolated yeast reductase to beer with subsequent forced aging resulted in a lower concentration of dicarbonyl compounds.196 Overexpression of an involved reductase resulted in beers with 30−40% lower concentrations of Strecker aldehydes.197[16]
Although sufficient oxygen must be supplied to yeast to promote lipid synthesis and satisfactory fermentation, it has been demonstrated that oxygen can lower the viability of yeast, exerting its effect via superoxide or species derived from it (20). Upon exposure to oxygen, yeast responds by synthesizing SOD and catalase, enzymes that are suppressed under anaerobic conditions (20). As for all aerobic organisms, those enzymes are triggered to promote protection against radical damages. In the transition period necessary for the elaboration of these enzymes, yeast is susceptible to oxygen radicals, a problem that should be considered when designing systems for providing oxygen to yeast.[17]
Preparing yeast for fermentation
Rehydrating dry yeast
- https://www.brunwater.com/articles/water-for-yeast-rehydration
- https://www.homebrewtalk.com/threads/dry-yeast-rehydration.681608/
Starters for liquid yeast Yeast produce membrane lipids only when grown aerobically. In the initial growth phase, proper oxygen management leads to proper production and storage of sterols in the yeast cell, which can be shared with subsequent daughter cells. It is possible to increase yeast ethanol tolerance by promoting synthesis of sterols, by adding oxygen (air) in the starter and during fermentation. Yeast lees deplete the oxygen content and can impact the redox potential and formation of VSCs.[18]
While oxidative stress is known to occur, is it significantly less that stress from carbon dioxide. High amounts of foam means that insufficient oxygen delivery is occurring.[19]
The oxygen content in the propagation medium has to exceed 0.15 ppm in order to allow good growth of the yeast and to guarantee pitching yeast with a good physiology.[20]
Stirring helps with oxygen diffusion[21]
- Smack Pack activation
- Hold it upside down at an angle and when the inner pouch sinks into the corner, gently squeeze, slowly increasing pressure, until you feel the inner pouch pop.[22]
Biomass may actually be more important that cell count with regard to pitch rate.[23] However this isn't easy to measure at home. Pitching rate calculators are still useful for determining correct pitch rate.
Yeast in worts rich in glucose may not be able to adapt to metabolize maltose and maltotriose, leading to slow or stuck fermentations.[24]
Of 157 sequenced strains of S. cerevisiae, the majority of strains selected for use in alcoholic beverages have lost cinnamic acid decarboxylation function. A variety of loss-of-function mutations are found in either Pad1, Fdc1, or both among beer, wine, and sake strains (POF−), however all strains sequenced that fall into “wild”, industrial, or bread baking groups retain POF activity. Among the strains sequenced are three Bavarian wheat beer strains, where POF+ activity is essential for the clove/spice character attributed to 4-VG.[25]
slow/stuck fermentation
In general, fermentation rates increase with temperature up to a maximum value (commonly >29C), after which premature cessation is probable due, in part, to elevated ethanol toxicity (D’Amato et al. 2006).[26] Beltran et al. demonstrated that low temperature fermentations altered nitrogen transport and metabolism, and suggested that coordination between carbon and nitrogen metabolisms may be hampered. Increased fermentation temperatures also alter the nutrient requirements of Saccharomyces (Shinohara et al. 1996; Ribéreau-Gayon et al. 2000), but these effects are not well defined for most nutrients.[26]
When brewer's yeast is exposed to high concentrations of glucose, a phenomenon referred to as the "glucose effect" may be experienced with poor quality yeast, which can result in sluggish and "hung" wort fermentations.[27]
The basis for premature yeast flocculation (PYF) is increasingly being identified as the result of specific fungal xylanase or pectinase activities which release relatively small (40–100,000 in molecular weight) arabinoxylans or pectins from the malt husk that bind to the lectin-like proteins on the yeast surface to bridge multiple yeast cells resulting in excessive flocculation (Koizumi et al., 2008; van Nierop et al., 2004).[28]
Nutrition Brewer's yeast strains cannot assimilate proteins and longer chain peptides due to the fact that cells hardly secrete proteases during brewing. The assimilable nitrogenous compounds for brewer's yeast are known as free amino nitrogen (FAN) which can be defined as the sum of FAA, ammonium ions, and to a lesser extent, di- and tripeptides.[29] The transport of FAA across the cell membrane is active, driven by the proton gradient via specific and general amino acid permeases. FAA have been categorized into four groups in ale yeast on the basis of their assimilation patterns (Jones and Pierce, 1964). In this model, group A is reported to be assimilated immediately after the yeast cells come into contact with wort and almost totally consumed after a few hours of fermentation. Group B is taken up more slowly, but assimilated gradually throughout fermentation. Group C is not utilized until group A have disappeared from the wort. Pro is the sole member of group D and is also the least preferred amino acid by brewer's yeast, because its dissimilation requires the presence of a mitochondrial oxidase which is inactive under anaerobic conditions. However, it has been proven that this assimilation pattern is often specific to the conditions employed and among them the yeast strain's nutritional preferences is perhaps more significant. Increasing the FAN has minor and somewhat unpredictable effects on yeast growth and attenuation.
Generally, nitrogen sources impact yeast cells in two ways, one by increasing the biomass production and the other by improving fermentability. Moreover, fermentation outcomes are affected not only by the concentration but also by the form of nitrogen source. For example, it has been reported that a shorter fermentation time is obtained when Glu is used in fuel ethanol production, while the effect is reversed when Gly is used.[29]
Deficits in FAN directly can lead to an insufficient and slow start of the fermentation, insufficient fermentation performance, and stuck fermentations.[30] Low molecular weight nitrogen compounds, especially amino acids, influence the metabolism of the yeast. In particular, they impact the production of higher alcohols and vicinal diketones.
"Nutrition" in this context refers to sources of yeast-assimilable nitrogen (YAN), necessary vitamins, and certain trace minerals. YAN is the amount of nitrogen from the combination of ammonium plus Free Amino Nitrogen (FAN), in the form of amino acids.
Wort produced from a high percentage of malt tends to supply necessary vitamins in levels well beyond what is needed for fermentation (including biotin, inositol, and pantothenate). The levels left after fermentation as to the overall nutritional value of beer. Malt also tends to add the required levels of copper, iron, zinc, and magnesium.[31]
Nitrogen is generally plentiful in wort and typically does not require supplementation for beer production.[2][32] The concentration of the amino acids isoleucine, valine, phenylalanine, glycine, alanine, tyrosine, lysine, histidine, arginine and leucine, are considered important, as these are an important part of the complex system regulating the biosynthesis of flavour-active compounds formed by yeast.[2] However, if supplementation is desired, a mixture of amino acids is more favorable to growth than when ammonium ions are the source of nitrogen.[2] Phenolic yeast may have a higher nitrogen requirement.[2]
Yeast consume at least 100-140ppm FAN in wort. Since proline cannot be utilized, wort has to contain 200-220ppm FAN. Inadequate nutrition can result in reduced yeast propagation and a delay in fermentation and maturation, and ultimately the retention of undesirable "young beer" off-flavors. Higher modified malts produce more FAN.[33] If adjuncts are used, the brewer should consider using a protein rest (45-50°C) (see Mashing) or adding yeast nutrient.
Worts that are prepared with reasonable percentages of malt tend to be rich in amino acids. Low FAN levels are undesirable in wort. The traditional rule is that serious problems (long lags, high diacetyl, etc) can result from FAN below 150-175ppm. A 12°P malt wort will typically have 225-275ppm FAN, which is ideal.[31] As a general rule, it is usually desirable to keep FAN levels below 350ppm, something that can be achieved with a suitable mashing schedule.
In worts from all-malt mashes the levels of amino acids are nearly always adequate for good yeast growth. However, in worts made using mash tun and/or copper adjuncts the FAN levels may fall below the 100–140 mg/litre level, which is regarded as the minimum needed for trouble-free fermentations.[24]
Too much or too little FAN can increase diacetyl production during fermentation.[31]
The minimal wort FAN level to achieve satisfactory yeast growth and fermentation performance in normal gravity wort fermentations (12°P) is 130 mg/L but, for rapid attenuation resulting in higher ethanol production, increased levels of wort FAN are required (170–190 mg/L). Meilgaard suggested that, during normal wort gravity fermentations, a minimum FAN level of 150 mg/L is required to permit rapid and complete attenuation. However, optimal wort FAN levels differ from fermentation to fermentation and from yeast strain to yeast strain; thus, they are considered controversial and unverified and are just guidelines. Furthermore, the optimum FAN values change with different wort sugar levels.[34] Wort FAN is affected by the malt/adjunct ratio, the mashing schedule, barley variety, barley growth conditions, and various malting parameters. Under high-gravity brewing conditions, brewing yeast strains need extra FAN to cope with increased osmotic stress, other stress conditions, and the additional yeast growth required for efficient wort fermentation. This means that, as wort gravity increases, the levels of assimilable nitrogen should also increase in order that a certain rate of glycolytic flux and high cell viability and vitality is maintained. This avoids incomplete fermentations. Finally, FAN is used to provide not only nitrogen to the yeast cells for growth but also the wort nitrogen content or its metabolic products which affect beer flavor compounds and overall stability.
yeast cells under conditions of high gravity brewing needed extra assimilable nitrogen to cope with osmotic stress and other stress conditions.[29]
See also: Protein
The levels of vitamins present in conventional (all-malt) worts rarely or never limit yeast growth and fermentation.[24]
Review:
- https://www.mdpi.com/2311-5637/3/3/41/pdf
- https://fenix.isa.ulisboa.pt/downloadFile/563022967865351/2005Implications%20of%20nitrogen%20nutrition%20for%20grapes,%20fermentation%20and%20wine.pdf
- https://books.google.com/books?id=bHuCdG5VSmUC&pg=PA92&lpg=PA92&dq=vitamin+e+wort+-john&source=bl&ots=8c_VpU3Fs4&sig=ACfU3U1fgQ3aPJpEANWLRXjbv580IWc1Zw&hl=en&sa=X&ved=2ahUKEwiq96Ouqp_oAhUGVa0KHf3bDJoQ6AEwA3oECAcQAQ#v=onepage&q=vitamin%20e%20wort%20-john&f=false
- https://www.mbaa.com/publications/tq/tqPastIssues/1997/Abstracts/tq97ab40.htm
Measuring YAN
See YAN testing
YAN Target
The optimal YAN level for wine is generally 250-350ppm (nitrogen).[35][36][37]
Nutrient tables:
Too much YAN (>350mg/L) can induce an overpopulation of yeast, which will increase stress conditions and produce undesirable characteristics such as off-flavors or stuck fermentation.[38] To ferment 1g/L of sugar, yeast need 1mg/L of YAN. (1°Brix = 10g/L sugar) For good population growth, a minimum of 150mg/L YAN is needed.
YAN target depends heavily on yeast strain and fermentation conditions (e.g. initial sugar, temperature, fermentation aeration).[37][36]
- YAN requirement for clean/fruity flavour has only been determined in Chardonnay: low YAN juices gave more complex aromas whereas moderate YAN gave cleaner and more fruity aromas in young wines.[37]
- Large additions of inorganic nitrogen (DAP) can increase risk of ester taint (ethyl acetate) formation.[37]
Proficient YAN guidelines should be based on starting Brix (Butzke and Dukes, 1998). For instance, a starting brix of 21 requires 200 mg N/L whereas as starting brix of 25 requires 300 mg N/L.[39]
DAP is 21% nitrogen by weight. Adding DAP during active fermentation will help the yeast remove existing sulfide within a few hours, but only if the ABV is under 7%.[35]
Ammonia (in the form of DAP) can prevent the appearance of aromatic degradation products from amino acids. Amino acids are an important source of yeast esters, which can add to complexity and wine quality. Thus, the supply of nitrogen must be available to allow a continuous re-synthesis of these proteins. If that does not occur, the yeast lose the ability to conduct the fermentation. Nitrogen addition may be effective in avoiding problem fermentations until about two-thirds of the sugar is utilized. Cells which have passed the point of transcriptional responsiveness will not respond to added nutrients.[18]
Equal proportions of ammonium to amino nitrogen and moderate initial concentrations of DAP (100 to 150 mg N/l) result in the lowest sulfide formation after peak fermentation.[40]
Vitamins and Trace Elements
- Thiamine
- Used as a co-enzyme for fermentation. It stimulates yeast growth, speeds up fermentation, and decreases undesirable fermentation byproducts, notably acetaldehyde.[41]
- Biotin
- Biotin is the most important vitamin for yeast (Fig.2) It is involved in almost all enzyme reactions that create the compounds yeast are made of: proteins, DNA, carbohydrates, and fatty acids. Biotin deficiency results in slow yeast growth and stuck fermentations.[42]
- Zinc
- It is needed in the micro molar (10-3M) range in wort. Zinc is important in the cell cycle (reproduction), and is a cofactor for alcohol dehydrogenase, the enzyme responsible for alcohol production. Other metal ions can not substitute in place of zinc. Supplementation of zinc into brewers worts generally has the effect of speeding up fermentation, as well as preventing stuck fermentations.[42]
Typical vitamin requirements for yeast include biotin, nicotinic acid, vitamin B, and pantothenic acid.[42]
Essential vitamins: 250 µg/l Ca-pantothenate, 250 µg/l thiamin a HCl, 25 µg/l pyridoxine, 2 µg/l biotin.[40]
0.2 mg/L folic acid, 200 mg/L myo-inositol, 4 mg/L pyridoxine, 4 mg/L nicotinic acid, 1 mg/L thiamin, 0.4 mg/L riboflavin and 0.250 mg/L pantothenic acid.[43]
Staggered Nutrients
- Higher initial juice/must YAN values increase fermentation rate and heat production.[37]
- DAP can be added in divided doses to give a more moderate rate of fermentation.[37]
- Higher initial juice/must YAN values or DAP additions can increase the risk of residual YAN in finished wines.[37]
Amino acids are brought into the yeast cell through transport across the cell membrane. The presence of alcohol and ammonium ions (i.e., DAP) inhibit amino acids from being brought into the cell. This is why winemakers are advised NOT to add DAP at inoculation or at the beginning of fermentation, as yeast can actively absorb organic nitrogen in the juice (aqueous) environment.[44] Once alcohol concentrations begin to increase, as a result of primary fermentation progression, transport of amino acids from the wine into the yeast cell will be inhibited. Therefore, the primary source of nitrogen will then come from inorganic sources, such as DAP.[44][citation needed] Higher concentrations of the inorganic component of YAN can lead to a high initial biomass of yeast. This is a problem because the rapid increase in yeast populations can lead to starvation by the majority of the yeast by mid- to late-fermentation, especially if there is not enough nutrition to fulfill all of the yeast during fermentation. Yeast starvation leads to yeast stress, and one of the stress responses by yeast is the production and release of hydrogen sulfide. Therefore, having a high YAN at the start of fermentation may cause hydrogen sulfide issues in the wine by the time fermentation is complete.[44]
Yeast don’t need all the nutrients at the same time:
- During the growth phase, yeast need vitamins, minerals and nitrogen. The presence of alcohol and/or ammonium ions inhibits transport of amino acids through cell membranes and reduces their consumption.[38]
- To optimize their absorption and efficiency, amino acids should be added at inoculation, before ammonium ions. At this stage, yeast can assimilate amino acids to build ‘healthy’ cells which are resistant to stress conditions and produce aromas.[38]
- At 1/3 of sugar depletion, yeast start to become stressed and the assimilation of nitrogen is lower. To complete fermentation and increase their alcohol resistance, they need fast and easy nutrients to absorb (ammonium ions) and survival factors (sterols and unsaturated fatty acids) with oxygen.[38]
- In case of strong nitrogen deficiency, must needs to be corrected by an addition of ammonium ions 24-48 hours after inoculation (after the addition of amino acids).[38]
- The nutrient additions should be split between inoculation and no later than 1/3 sugar depletion.[38]
- Late nutrient additions are ineffective for yeast activity and can promote development of spoilage organisms, appearance of off-flavors and formation of biogenic amines.[38]
Nutrient Products
- https://morewinemaking.com/products/fermfed-yeast-nutrient.html
- https://catalogapp.lallemandwine.com/uploads/nutrient/docs/1b340d1ae3fc0a693339355555cdfcfa4971a1e4.pdf
An innovative new method of mineral delivery is Servomyces, which White Labs represents in North America. It is produced in a patented process, by which brewers yeast is grown in the presence of metal ions, including zinc and magnesium, and then dried and killed. When the dry, dead yeast (Servomyces) is added to brewery fermentations (in very minute quantities), the effect is dramatic to fermentation speed and to yeast performance/viability. [45]
- https://onlinelibrary.wiley.com/doi/pdf/10.1002/j.2050-0416.2011.tb00472.x
- Handbook of Brewing section 10.4
- Free Amino Nitrogen in Brewing - MDPI
- https://www.brewersjournal.info/free-amino-nitrogen/
Flavor compounds During active biomass accumulation, H2S and ester production may occur. In this case, ester formation is stimulated by the presence of nitrogen, indicating that biosynthetic reactions are the source of these compounds. Once active growth has diminished and ethanol is accumulating, amino acid degradation occurs and, at this time, additional esters and fusel compounds may be produced. The fusel compounds come from the degradation of amino acids as nitrogen sources via the Ehrlich pathway.[46] https://wineserver.ucdavis.edu/industry-info/enology/fermentation-management-guides/wine-fermentation/characters -- discussion of VSCs, esters, and aldehydes
If the total fusel alcohol level is below 300 mg/L, the wine is described as fruity and pleasant containing peach and apricot aromas. Above 400 mg/L, the wine becomes pungent with a strong foul chemical taste and aroma. In wine, the total produced varies within this range, from less than 100 to greater than 500 mg/L. The individual compounds vary from 10-140 mg/L. The amounts formed show a strong stain dependence.[46]
in general, when acetaldehyde is found in wine it comes from the chemical re-oxidation of ethanol during aging and oxygen exposure of the wine. Acetaldehyde levels in wine range from 1 – 160 mg/L. It has a putative threshold of 100 mg/L. It is described most often as sherry-like in wines, but has bruised apple and nutty notes as well. The higher aldehydes derived from amino acids can have strong nutty and rancid nutty notes at high concentrations and are made under the same chemical conditions conducive to acetaldehyde formation. At lower concentrations, they may confer notes of coffee, chocolate or stone fruits.[46]
With respect to ethyl acetate formation, the amounts made by Saccharomyces are inconsequential but the amounts formed by the acetic acid bacteria and Hanseniaspora uvarum can be so high that these compounds will not be lost from the wine during fermentation or subsequent processing. If the odor of ethyl acetate is noticed during a cold soak or other pre-fermentation treatment, that treatment should be halted and the fermentation inoculated immediately.[46]
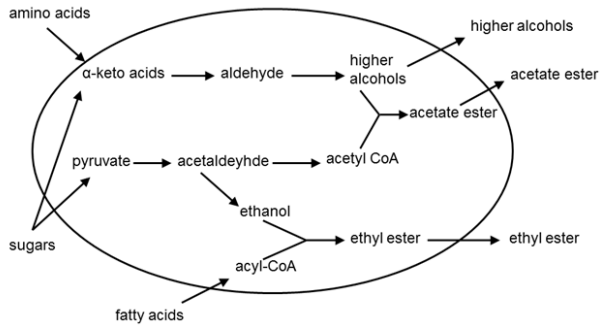
Fusel alcohols are produced from the carbon skeletons of amino acids, and the effect of fusel alcohols on the finished product can be quite negative if present above or near their respective flavor thresholds.[31]
In many breweries producing South- ern German-style wheat beer, otherwise known as weissbier, after the installation of new cylindroconical fermentors, it is common for the beers to exhibit a noticeable decline in the bouquet characteristic of the style, which consists of primarily of compounds like isoamyl acetate (banana ester)[2]. The reason behind this somewhat diminished weissbier aroma is, among others, the high rate of yeast reproduction, which reduces the amount of the acetyl-coenzyme A available for ester formation. In addition, the high hydrostatic pressure in vertical vessels moderates the production of higher alcohols, thus reducing the numbers of reactants for the formation of esters. In short, the higher the liquid level is in a fermentation tank, the stronger the convection and homogenization, which results in a reduction in the formation of esters (fig. 4).[47]
The estery notes in beer have been observed to become more pronounced as the ratio of glucose to maltose tips in favor of glucose.[47] Alcoholic fermentation with yeast in the presence of high concentrations of glucose leads to a delay in the onset of maltose metabolism after an initial rapid decline in the extract content of the wort (similar to a "second lag phase"). This explains the plateau in the extract curve. During this time, the yeast are scarcely reproducing and are compensating with the synthesis of maltose permease and maltase. The diminished yeast reproduction results in overflow of the acetyl-CoA pool and thus greater ester formation and fruitier beers.
Yeast fermentation of maltose is repressed by glucose.[48]
This also comes into play when using adjuncts in brewing.
In many Belgian-style specialty beers, POF+ S. cerevisiae strains are required to impart spice notes in the finished beer.[25] There is a wide variety among these strains regarding POF activity. This at least partially explains the difference in volatile flavor compounds (phenolics and esters) produced by different strains such as those utilized for weissbier vs Belgian tripel styles for example.
Only a few commercially available brewing yeast strains specifically list “peppery” as an expected descriptor for the finished beer. These include White Labs WLP565, Wyeast 3711, Wyeast 3726, BSI S-11, BSI S-26, and BSI 565. All of these strains are identified by the supplier as a most suitable for saison-style beers.[25]
Certain undesirable ethyl esters, heterocyclic compounds and carbonyl compounds are produced during fermentation without oxygen involved [1].[49]
See:
- https://www.mdpi.com/2311-5637/4/2/23/pdf
- https://www.homebrewtalk.com/threads/be-256-dry-fermetis-abbey-has-anyone-used-it-results.670423/
- Overbuild starters
- Drying kveik
- Under beer, jars vs vials
- Isotonic saline
- Slants or plates
- Temperature
- Periodic feeding
Molecular oxygen is taken up by yeast at the start of the fermentation and is used by the cell to synthesise sterols and unsaturated fatty acids which are essential components of the yeast’s membrane. The need for oxygen can be removed if sterols (e.g. ergosterol) and unsaturated fatty acids (e.g. oleic acid) are added directly to the wort. In terms of releasing energy, aerobic respiration is more efficient than anaerobic respiration. However in yeast the temptation to use the available oxygen for aerobic respiration is suppressed through a mechanism described as the Crabtree effect. In the presence of glucose sugars (above 1% by weight) yeast (Saccharomyces spp) uses glucose to produce alcohol and uses the oxygen to produce the necessary lipid compounds. The presence of insufficient lipid compounds will lead to a defective fermentation due to inadequate yeast cell reproduction, which in turn will lead to:[50] • Slow and sticking fermentations • Off flavours – e.g. poor removal of diacetyl and acetaldehyde • Poor yeast crop in terms of quantity and vitality • Low ester formation Excess oxygen will lead to: • Rapid fermentations • Excessive yeast growth and hence beer losses • Higher ester production – giving fruitier flavoured beers
yeast strains significantly affected the antioxidant level of beer. The antioxidant value of beer with yeast strain C was higher than that of the other two strains and its flavor stability was the best among these three beers. It was very interesting that there was no correlation between the fermentation time and the antioxidant level. Sulfite content in beer is also shown in Table II. The results show that the effect of yeast strain on beer flavor stability is mainly due to the ability of yeast to produce sulfite. These results suggest that the selection of yeast strain is one of the important factors for improvement of beer flavor stability. Similar suggestions have been also made previously by Narziss et al (13).[51]
Pitching rates also had an effect on the EA (endogenous antioxidant) value of finished beer (Table IV). The EA value of beer increased with pitching rates, while the fermentation time significantly decreased. In the case of 54 × 106 cells/ml, the EA value of beer significantly increased, but the profile of volatile compounds in beer was rather different from the others (data not shown). In the range of pitching rates conventionally used, the effect of pitching rates on the EA value of beer was considered not great. The sulfite content in beer slightly increased with pitching rates. The effect of pitching rates on the EA value might be caused partly by the differences in the sulfite content of beer, although the EA value was not necessarily in proportion to the sulfite contents. Fermentation temperature had an effect on the EA value of finished beer (Table V). Fermentation temperatures of 9, 12, and 15°C were tested. The EA value of these beers was almost the same, although the fermentation time was significantly decreased with the rise in temperature. Although the data is not shown here, in the case of beer brewed with another yeast strain, a different result was observed: The EA value of beer brewed at a low fermentation temperature had the tendency of having higher sulfite content and higher EA value than those of the beer brewed at a high temperature. The reports on temperature dependency of sulfite production during fermentation were different among some researchers (2,15,19). It seemed that the effect of fermentation temperature on sulfite production might be different based on the physiology of the yeast strains used. Thus, the effect of fermentation temperature on beer flavor stability seemed to be different among the yeast strains used. Lustig et al, on the other hand, reported that higher temperature during the primary fermentation might be harmful from the view of residual concentration of some aging aldehydes (11). These results suggested that the selection of fermentation temperature for improving beer flavor stability must also be made after careful consideration. To clarify where there is a simple relationship between EA value and sulfite concentration of beer, or not, the plot of EA values against sulfite concentration is shown using the data from these various fermentation experiments (Fig. 4). These results show that sulfite is one of the essential and important determinants (antioxidants) of EA value, but other factors may also influence EA value.[51]
YAN has the most impact on fermentation speed compared to other compounds. It impacts yeast biomass at the beginning of fermentation and sugar transport during fermentation. At the end of growth phase, N is depleted resulting in decreased protein synthesis and sugar transport. A YAN addition at this point reactivates protein synthesis and sugar transport increasing the fermentation rate. Oxygen is rapidly consumed in the beginning of fermentation. Decreased oxygen inhibits sterols and fatty acid synthesis by yeast. This causes decreased yeast growth and viability at the end of fermentation.[52]
Sterols and fatty acids are survival factors needed for the yeast cell membrane to function. As ethanol increases, hydrogen ions accumulate in cell requiring more energy to expel them. The pH decreases inside the cell causing cell death. Oxygen adds at end of growth phase increase sterol production. Therefore, microoxygenation and pump overs would be beneficial at 1/3 of the way through alcoholic fermentation (end of yeast growth phase).[52]
N assimilation: The manner in which N is assimilated by yeast depends on the source. Organic N (amino acids) is actively transported into the yeast cell. Through additional reactions N is incorporated into glutamine and glutamate and eventually used in the synthesis of other amino acids and nitrogenous compounds. This process is gradual and efficient compared to inorganic sources. Ammonium nitrogen (inorganic N) is consumed quickly and is less beneficial. Amino acid mixtures vs single N sources are more efficient because the yeast directly incorporates the amino acids into proteins rather than having to synthesize them. Ammonia, which exists as ammonium (NH4+) ions in must, is used by yeasts prior to amino acids. The presence of NH4+ delays timing and uptake of amino acids by yeast. The timing of N supplements and form of supplement will impact fermentation and volatiles. Types of N supplements include Diammonium phosphate (DAP), proprietary blends of DAP and amino acids (e.g. Superfood®, Fermaid K®, Actiferm) and balanced nutritional formulas containing inorganic N (e.g. Fermaid O®), organic N, sterols, yeast cell walls, fatty acids, yeast autolysis products and others. DAP is best used with low N musts. Other balanced nutrients should be added as well. At a rate of 100 mg/L DAP, 20 mg/L YAN is added.[52]
Juice/must can be vitamin deficient as well as deficient in assimilable nitrogen when there is a high incidence of microorganisms (mold, yeast and/or bacteria). Growth of Kloeckera apiculata has been reported to rapidly reduce thiamine levels below those required by Saccharomyces sp. (18). Further, the use of SO2 may lead to additional reductions in levels of thiamine (15). Saccharomyces sp. has been shown to synthesize all required vitamins, with the exception of biotin. However, vitamin supplementation has been demonstrated to be stimulatory (19). Thus, it is usually desirable to add a mixed vitamin supplement with the nitrogen additions.[53]
Pekur, et al. (24) reported that, at increased pressures, carbon dioxide reduces the yeast’s uptake of amino acids.[53]
Maltose(100 g) + aminoacids(0.5 g) → yeast(5 g) + ethanol(48.8 g) + CO 2 (46.8 g) + energy(50 kcal)[54]
The ability to produce high concentrations of ethanol without permanently damaging yeast cells and compromising fermentation performance is particularly important in brewing, as yeast populations are normally required to ferment multiple brews in succession192. While ethanol toxicity has been ascribed to non-specific effects on the cell48,103, the weight of evidence suggests that membranes are the main targets of ethanol toxicity. It has been suggested that the effect of ethanol on membranes is due to its insertion into the hydrophobic interior and the resultant effects on polarity, exchange of polar molecules and the position of membrane proteins91. Direct ethanol exposure results in increased fluidity of membranes103,127,145 and increased membrane permeability causing leakage of amino acids, leakage of compounds absorbing light at 260 nm and leakage of proteases75,91,146,182 and influx of protons99,117,120. The cellular membrane is not, however, the only target for ethanol toxicity and the mitochondrial membrane may also suffer damage, leading directly or indirectly to mitochondrial DNA damage and the generation of respiratory deficient (petite) strains40,75,99. Indeed, serial repitching of yeast in breweries has been associated with an increase in the frequency of petite mutants, probably caused by repeated exposure to potentially toxic levels of ethanol during fermentation and storage98[55]
Yeast cells have a very high growth demand for Mg2+, but not for Ca2+[56]
The requirement of trace elements by yeast can be classed into three categories:[57] macroelements (K*. Mg2\ Ca2\ Zn2\ Fe2* '\Mn2*, Cl); microelements (Co=\B2>, Cd2*, Cr3*-6*, Cu2', I, Mo2*, Ni2*, Va-") and inhibitors (Ag\ As3*, Hg+, Li*. Ni2\ Os2\ Pd\ Se4*, Te4'). However, when considering the ionic nutrition of yeast fermentation, it is the concentrations of K\ Co2*, Mg2* and Zn2* that are the most critical factors.
Zinc, magnesium and calcium are the most essential metal ions in wort. They must be present in sufficient amounts to ensure optimal yeast performance and ethanol yield.[58]
Minerals such as zinc, manganese, magnesium, and calcium are found in trace amounts in worts and are required for adequate fermentation performance. A poor quality barley crop can give rise to worts that are deficient in these metals. This, in turn, can lead to inconsistencies in the process and product, including lagging fermentations and poor yeast quality.[59]
The most important metals that influence yeast fermentation processes are potassium and magnesium (as bulk metals), and calcium, manganese, iron, copper and zinc (as trace metals).[60]
The major stresses encountered by industrial yeasts are temperature shock, osmotic stress and ethanol toxicity.[60]
In the brewing industry, the viability and vitality of pitching yeast are crucially important for continued successful beer-making. Yeast management before, during, and after fermentation should endeavor to minimize physiological stresses imparted on brewing yeast cultures (21,27). Stress may be imposed on brewing yeast at pre-fermentation (e.g., acid-washing, cold-shock, oxidative stress, and nutrient starvation); primary and secondary fermentation (e.g., osmostress, ethanol toxicity, pH/temperature fluctuations, and CO2/hydrostatic pressure); and post-fermentation (e.g., mechanical shear, cold-shock, and nutrient starvation). Several bioprocessrelated approaches have been advocated to maximize brewing yeast viability and vitality at different stages of yeast handling (13,31). These include nutrient-controlled yeast propagation by fed-batch cultivation, selective yeast cropping from cylindroconical fermenters, and strict control over yeast storage and acid-washing conditions.[61]
yeast requires a minimal amount of inorganic-phosphate, potassium, and magnesium (250, 500, and 70 mg/L, respectively) to support yeast-growth and ethanol/flavour formation. Inorganic-phosphate was important for fatty acid esters formation/short chain fatty acid (SCFA) reduction. Potassium was important in the formation of acetate esters/higher alcohols. Magnesium was the most important inorganic element for ester formation/SCFA reduction; furthermore, ethanol production is magnesium-dependent.[62]
Articles to be reviewed[edit]
- https://onlinelibrary.wiley.com/doi/pdf/10.1002/jib.242
- https://onlinelibrary.wiley.com/doi/pdf/10.1002/j.2050-0416.2007.tb00259.x
- https://onlinelibrary.wiley.com/doi/full/10.1002/jib.184
- https://onlinelibrary.wiley.com/doi/full/10.1002/jib.145
- https://onlinelibrary.wiley.com/doi/10.1002/jib.104
- https://www.ros.hw.ac.uk/bitstream/handle/10399/3521/JoseyM_0318_eps.pdf?sequence=1&isAllowed=y
- "Effect of Fermentation Conditions on Staling Indicators in Beer"
- "Maltotriose utilization in lager yeast strains: MTT1 encodes a maltotriose transporter"
- Kunze section 1.4
- https://www.sciencedirect.com/topics/agricultural-and-biological-sciences/fermentation
- Textbook: brewing microbiology 3rd edition 2002
- Verbelen, P. J., and Delvaux, F. R. Brewing yeast in action: Beer fermentation. In: Applied Microbiology. M. K. Rai and P. D. Bridge, Eds. CAB International, Oxon, UK. Pp. 110-135, 2009.
- Microorganisms in Fermented Apple Beverages: Current Knowledge and Future Directions
- Effects of hydrostatic high pressure on microbiological and technological characteristics of beer
- http://www.themodernbrewhouse.com/wp-content/uploads/2017/04/poeschl_0807.pdf The Influence of Fermentation-Control on the Colloidal Stability and the Reducing Power of the Resulting Bottom Fermented Beers
- Krogerus, K. and Gibson, B.: A re-evaluation of diastatic Saccharomyces cerevisiae strains and their role in brewing. Applied Microbiology and Biotechnology, 104 (2020), pp. 3745-3756.
- https://www.biorxiv.org/content/10.1101/2020.06.26.166157v1.full
- http://beer.suregork.com/sugar_utilization_by_yeast_during_fermentation.pdf
- https://sfamjournals.onlinelibrary.wiley.com/doi/pdf/10.1111/j.1365-2672.2004.02472.x
- https://onlinelibrary.wiley.com/doi/full/10.1002/jib.145
- Novel Non-Cerevisiae Saccharomyces Yeast Species Used in Beer and Alcoholic Beverage Fermentations
- Hill, Stewart - Free Amino Nitrogen in Brewing
- https://www.tandfonline.com/doi/abs/10.1094/ASBCJ-2013-0921-01 Stewart, G. G., Hill, A. E., and Lekkas, C. Wort FAN—its characteristics and importance during fermentation. J. Am. Brew. Chem. 71:179-185, 2013.
- Verbelen, P.J., Delvaux, F.R., 2009. Brewing yeast in action: beer fermentation. Applied Mycology 110–135.
- Fleet, G.H., 2003. Yeast interactions and wine flavour. International Journal of Food Microbiology 86, 11–22.
- Fleet, G.H., 2008. Wine yeasts for the future. FEMS Yeast Research 8, 979–995.
- Romano, P., Fiore, C., Paraggio, M., Caruso, M., Capece, A., 2003. Function of yeast species and strains in wine flavour. International Journal of Food Microbiology 86, 169–180.
- Guido, L.F., Rodrigues, P.G., Rodrigues, J.A., Gonçalves, C.R., Barros, A.A., 2004. The impact of the physiological condition of the pitching yeast on beer flavor stability: an industrial approach. Food Chem. 87, 187–193.
- Saison, D.; De Schutter, D.P.; Delvaux, F.F.R.; Delvaux, F.F.R. Improved flavor stability by aging beer in the presence of yeast. J. Am. Soc. Brew. Chem. 2011, 69, 50–56.
- Arão Cardoso Viana, Tatiana Colombo Pimentel, Rafaela Borges do Vale, Lorena Santos Clementino, Emilly Thayná Januario Ferreira, Marciane Magnani, Marcos dos Santos Lima. American pale Ale craft beer: Influence of brewer's yeast strains on the chemical composition and antioxidant capacity. LWT 2021, 152 , 112317.
- Phenolic off-flavour problems caused by saccharomyces wild yeast.
- The biotransformation of simple phenolic compounds by Brettanomyces anomalus
- Bioflavoring by non-conventional yeasts in sequential beer fermentations
- Formation of 4-vinyl and 4-ethyl derivatives from hydroxycinnamic acids: Occurrence of volatile phenolic flavour compounds in beer and distribution of Pad1-activity among brewing yeasts
- Assessing Population Diversity of Brettanomyces Yeast Species and Identification of Strains for Brewing Applications
- CLONING OF A YEAST GENE WHICH CAUSES PHENOLIC OFF-FLAVOURS IN BEER
- Distribution of phenolic yeasts and production of phenolic off-flavors in wine fermentation
- Molecular and biochemical aspects of Brettanomyces in brewing
- Chatonnet, P., Dubourdieu, D., Boidron, J. N., & Lavigne, V. (1993). Synthesis of volatile phenols by Saccharomyces cerevisiae in wines. Journal of the Science of Food and Agriculture, 62(2), 191–202.
- Chapter 17 - Impact of yeast and bacteria on beer appearance and flavour in: Brewing Microbiology
- Schwarz, K. J., Boitz, L. I., & Methner, F.-J. (2012a). Enzymatic formation of styrene during wheat beer fermentation is dependent on pitching rate and cinnamic acid content. Journal of the Institute of Brewing, 118(3), 280–284.
- Gharwalova, L.; Sigler, K.; Dolezalova, J.; Masak, J.; Rezanka, T.; Kolouchova, I. Resveratrol suppresses ethanol stress in winery and bottom brewery yeast by affecting superoxide dismutase, lipid peroxidation and fatty acid profile. World J. Microbiol. Biotechnol. 2017, 33, 205.
- Technological Approach to Improve Beer Flavor Stability: Adjustments of Wort Aeration in Modern Fermentation Systems Using the Electron Spin Resonance Method
- "Effect of pitching yeast and wort preparation on flavor stability of beer"
- Decrease of Aged Beer Aroma by the Reducing Activity of Brewing Yeast
- Mochaba FM, O'Connor‐Cox ES, Axcell BC. A novel and practical yeast vitality method based on magnesium ion release. J Inst Brew. 1997;103(2):99–102.
- https://www.academia.edu/80000252/Beer_Molecules_and_Its_Sensory_and_Biological_Properties_A_Review
References[edit]
- ↑ Hill AE, Stewart GG. Free amino nitrogen in brewing. Fermentation. 2019;5(1).
- ↑ a b c d e Ferreira, Inês M., and Guido, Luís F. "Impact of Wort Amino Acids on Beer Flavour: A Review." Fermentation. 2018, 4, 23.
- ↑ a b https://onlinelibrary.wiley.com/doi/pdf/10.1002/j.2050-0416.2010.tb00425.x
- ↑ a b Kunze, wort production
- ↑ Guerra NP, Torrado-Agrasar A, López-Macías C, et al. Use of Amylolytic Enzymes in Brewing. In: Preedy VR, ed. Beer in Health and Disease Prevention. Academic Press; 2009:113–126.
- ↑ MacGregor AW, Bazin SL, Macri LJ, Babb JC. Modelling the contribution of alpha-amylase, beta-amylase and limit dextrinase to starch degradation during mashing. J Cereal Sci. 1999;29(2):161–169.
- ↑ Yu W, Zhai H, Xia G, et al. Starch fine molecular structures as a significant controller of the malting, mashing, and fermentation performance during beer production. Trends Food Sci Technol. 2020;105:296–307.
- ↑ Kerr ED, Fox GP, Schulz BL. Grass to glass: Better beer through proteomics. In: Cifuentes A, ed. Comprehensive Foodomics. Elsevier; 2020:407–416.
- ↑ Wu MJ, Clarke FM, Rogers PJ, et al. Identification of a protein with antioxidant activity that is important for the protection against beer ageing. Int J Mol Sci. 2011;12(9):6089–6103.
- ↑ a b Wu MJ, Rogers PJ, Clarke FM. 125th anniversary review: The role of proteins in beer redox stability. J Inst Brew. 2012;118(1):1–11.
- ↑ Golston AM. The impact of barley lipids on the brewing process and final beer quality: A mini-review. Tech Q Master Brew Assoc Am. 2021;58(1):43–51.
- ↑ a b c d Yang D, Gao X. Research progress on the antioxidant biological activity of beer and strategy for applications. Trends Food Sci Technol. 2021;110:754-764.
- ↑ Piper D, Jennings S, Zollo T. Pro-tips on lager decoction mashing, infusion mashing, yeast handling & sauergut (video). YouTube. Published 2022. Accessed 2024.
- ↑ Liguori L, De Francesco G, Orilio P, Perretti G, Albanese D. Influence of malt composition on the quality of a top fermented beer. J Food Sci Technol. 2021;58:2295–2303.
- ↑ Nielsen H. The control of oxygen in beer processing. J Inst Brew. 1973;79(2):147–154.
- ↑ Baert JJ, De Clippeleer J, Hughes PS, De Cooman L, Aerts G. On the origin of free and bound staling aldehydes in beer. J Agric Food Chem. 2012;60(46):11449–11472.
- ↑ Bamforth CW, Muller RE, Walker MD. Oxygen and oxygen radicals in malting and brewing: a review. J Am Soc Brew Chem. 1993;51(3):79–88.
- ↑ a b Zoecklein B. Enology notes #133. Wine/Enology Grape Chemistry Group at Virginia Tech. Published 2007. Accessed 2020.
- ↑ https://www.mbaa.com/publications/tq/tqPastIssues/2005/Abstracts/TQ-42-0128.htm
- ↑ Van Landschoot, A., et al. "Effect of pitching yeast preparation on the refermentation of beer in bottles." Cerevisia, vol. 29, no. 3, 2004, pp. 140–146.
- ↑ https://www.cmbe.engr.uga.edu/bche4510/assign/Interlude.pdf
- ↑ https://www.homebrewtalk.com/forum/threads/smack-pack-issues.676389/#post-8813147
- ↑ "Wiki Kwiki #005 - Lance Shaner of Omega Yeast Labs" (at ~30 minutes) Milk the Funk podcast, December 2019.
- ↑ a b c Briggs DE, Boulton CA, Brookes PA, Stevens R. Brewing Science and Practice. Woodhead Publishing Limited and CRC Press LLC; 2004.
- ↑ a b c Lentz M. The impact of simple phenolic compounds on beer aroma and flavor. Fermentation. 2018;4(1):20.
- ↑ a b https://onlinelibrary.wiley.com/doi/pdf/10.1111/j.1745-4557.2010.00365.x
- ↑ Stewart GG. Adjuncts. In: Stewart GG, Russell I, Anstruther A, eds. Handbook of Brewing. 3rd ed. CRC Press; 2017.
- ↑ https://www.sciencedirect.com/science/article/abs/pii/S0733521009000423
- ↑ a b c Lei H, Zheng L, Wang C, Zhao H, Zhao M. Effects of worts treated with proteases on the assimilation of free amino acids and fermentation performance of lager yeast. Int J Food Microbiol. 2013;161(2):76–83.
- ↑ Pahl R, Meyer B, Biurrun R. Wort and Wort Quality Parameters. In: Bamforth CW, ed. Brewing Materials and Processes: A Practical Approach to Beer Excellence. Academic Press; 2016.
- ↑ a b c d Fix, George. Principles of Brewing Science. 2nd ed., Brewers Publications, 1999.
- ↑ Jones BL, Budde AD. How various malt endoproteinase classes affect wort soluble protein levels. J Cereal Sci. 2005;41(1):95–106.
- ↑ Kunze, Wolfgang. "3.2 Mashing." Technology Brewing & Malting. Edited by Olaf Hendel, 6th English Edition ed., VBL Berlin, 2019, p. 230.
- ↑ Lekkas C, Hill AE, Stewart GG. Extraction of FAN from malting barley during malting and mashing. J Am Soc Brew Chem. 2014;72(1):6–11.
- ↑ a b Kaiser, K. "Controlling Reductive Wine Aromas." Brock University CCOVI lecture series. 1 Feb 2010.
- ↑ a b "Approximate YAN Contribution for the Important Yeast Nutrients."
- ↑ a b c d e f g "Yeast Assimilable Nitrogen (YAN)." The Australian Wine Research Institute. Accessed online March 2020.
- ↑ a b c d e f g "Yeast Nutrition for a Successful Fermentation." Enartis Vinquiry. Technical Harvest Newsletter. Volume 4. September 2014.
- ↑ https://midwestwinepress.com/2014/11/01/back-basics-preventing-rotten-eggs-aka-reduction/
- ↑ a b Butzke, CE and Park, SK. "Impact of Fermentation Rate Changes on Potential Hydrogen Sulfide Concentrations in Wine." J. Microbiol. Biotechnol. 2011. 21(5). pp. 519–524
- ↑ https://pennsylvaniawine.com/wp-content/uploads/2017/04/Yeast-Nutrition.pdf
- ↑ a b c White, C. "Yeast Nutrients Make Fermentations Better."
- ↑ Bohlscheid, JC., et al. "The influence of nitrogen and biotin interactions on the performance of Saccharomyces in alcoholic fermentations." Journal of Applied Microbiology. 102 2007. 390-400.
- ↑ a b c Gardner, DM. "Starting your fermentation right: nutrient supplementation." Penn State Extension Wine & Grapes U. 2016.
- ↑ https://www.jstrack.org/brewing/Yeast_nutrition_article.pdf
- ↑ a b c d https://wineserver.ucdavis.edu/industry-info/enology/fermentation-management-guides/wine-fermentation/characters
- ↑ a b Sacher B, Becker T, Narziss L. Some reflections on mashing – Part 2. Brauwelt International. 2016;6:392-397.
- ↑ Meussdoerffer F, Zarnkow M. Starchy raw materials. In: Esslinger HM, ed. Handbook of Brewing: Processes, Technology, Markets. Weinheim, Germany: Wiley-VCH Verlag GmbH & Co. KGaA; 2009.
- ↑ Wu MJ, Clarke FM, Rogers PJ, et al. Identification of a protein with antioxidant activity that is important for the protection against beer ageing. Int J Mol Sci. 2011;12(9):6089–6103.
- ↑ O'Rourke T. The role of oxygen in brewing. Brewer International. 2002;2(3):45–47.
- ↑ a b Uchida M, Ono M. Technological approach to improve beer flavor stability: analysis of the effect of brewing processes on beer flavor stability by the electron spin resonance method. J Am Soc Brew Chem. 2000;58(1):8–13.
- ↑ a b c Kelly M. Why, when, and how to measure YAN. Penn State Extension Wine & Grapes University website. 2020. Accessed online March 2024.
- ↑ a b Gump BH, Zoecklein BW, Fugelsang KC. Prediction of prefermentation nutritional status of grape juice: The formol method. Food microbiology protocols. 2001:283-96.
- ↑ https://www.academia.edu/30908633/Process_modelling_and_technology_evaluation_in_brewing
- ↑ Gibson BR. 125th anniversary review: improvement of higher gravity brewery fermentation via wort enrichment and supplementation. J Inst Brew. 2011;117(3):268–284.
- ↑ Walker GM, Birch RM, Chandrasena G, Maynard AI. Magnesium, calcium, and fermentative metabolism in industrial yeasts. J Am Soc Brew Chem. 1996;54(1):13–18.
- ↑ Rees EM, Stewart GG. The effects of increased magnesium and calcium concentrations on yeast fermentation performance in high gravity worts. J Inst Brew. 1997;103(5):287–291.
- ↑ Kordialik‐Bogacka E, Bogdan P, Ciosek A. Effects of quinoa and amaranth on zinc, magnesium and calcium content in beer wort. Int J Food Sci Technol. 2019;54(5):1706–1712.
- ↑ Bromberg SK, Bower PA, Duncombe GR, et al. Requirements for zinc, manganese, calcium, and magnesium in wort. J Am Soc Brew Chem. 1997;55(3):123–128.
- ↑ a b Walker G, De Nicola R, Anthony S, Learmonth R. Yeast-metal interactions: impact on brewing and distilling fermentations. In: Proceedings of the Institute of Brewing & Distilling Asia Pacific Section 2006 Convention. 2006.
- ↑ Walker GM. Magnesium as a stress-protectant for industrial strains of Saccharomyces cerevisiae. J Am Soc Brew Chem. 1998;56(3):109–113.
- ↑ Ribeiro-Filho N, Linforth R, Bora N, Powell CD, Fisk ID. The role of inorganic-phosphate, potassium and magnesium in yeast-flavour formation. Food Res Int. 2022;162:112044.