Ascorbic acid


Please check back later for additional changes
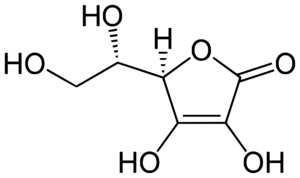
Ascorbic acid (AA), also known as Vitamin C, is an organic acid that is naturally present in some fruits, including oranges.
AA is an anti-oxidant.[1] It is used in low oxygen brewing to provide active oxygen scavenging. For this usage, it is suggested to use the same concentration of AA as sodium metabisulfite in the mash.
AA interferes with certain methods of SO2 testing, because it reacts with iodine.[2]
AA may be added at packaging of beer or wine in combination with sulfite.[3] Suggested dosage is 0.02-0.08 mg/L (ppm).
AA can be used to remove chlorine compounds from municipal tap water, but it should not be used in brewing for this purpose because the result of the reaction is oxidized ascorbic acid, which is a powerful oxidant and can potentially damage the wort.[4]
The shelf life of AA is generally at least 1-2 years.[5][6]
Ascorbic acid can increase the potential for wine oxidation if adequate sulfite is not present.[7]
Reducing agents must be coupled to the oxidation process in beer in order to recycle the transition metal ions through their lower, oxygen-active redox states. Ascorbic acid has the ability to perform this role during the Fe-catalyzed oxidation of ethanol in model reactions.[8]
Passivity in stainless steel is destabilized in reducing acids.[9]
Although vitamin C is present in green malt, it destroyed during kilning.[10]
Ascorbic acid (1.5 mg/g of barley flour) is effective at preventing browning.[11] -- better than sodium sulfite (0.1 mg/g of barley flour).
ascorbic acid remarkably protects tannoids from oxidation (tested 50mg ascorbate + 2.5g malt + 50mL H2O), even with high-oxygen brewing at that concentration.[12]
Several phenolic compounds in wort and beer are more potent antioxidants than ascorbic acid (vitamin C).[13][14]
The reversibly oxidized form of ascorbic acid (called dehydroascorbic acid) undergoes an irreversible change in aqueous solution above pH 4 at ordinary temperatures. The product of this change is a stronger acid than dehydroascorbic acid, and is a more powerful reducing agent than ascorbic acid itself. The rates of appearance of all of these manifestations of the irreversible change in dehydroascorbic acid exhibit the same dependence on the hydrogen ion concentration. They are also all independent of the presence of air or oxidizing agents. The irreversible change is therefore not an oxidation. It is also independent of the oxidizing agent used to form dehydroascorbic acid.[15]
Ascorbic acid can be made to take up the equivalent of at least 3 atoms of oxygen in the course of its oxidation, in three separate steps, depending on pH.[15]
Dehydroascorbic acid is restored practically quantitatively to ascorbic acid by H2S in acid solution. After its conversion to diketogulonic acid this property is lost. However, reduction becomes more rapid as pH increases.[15]
Surveys by different methods show that strains of yeast vary widely in their ability to produce H2S, thereby making the yeast strain the most important factor in determining H2S production (Rankine 1968a, Eschenbruch et al. 1978, Thornton and Bunker 1989, Thomas et al. 1993, Giudici and Kunkee 1994, Jiranek et al. 1995c, Spiropoulis et al. 2000, MendesFerreira et al. 2002). However, the characterisation of strains has proven technically problematic due to poor understanding of the regulation of sulfur metabolism in yeast. Recent studies by L. Bisson at UC Davis have suggested that no one method is suitable for determining the potential of a strain to produce H2S under winemaking conditions (Spiropoulis et al. 2000). This is largely due to the fact that the regulation of sulfur and nitrogen metabolism in yeast is complex (Mountain et al. 1991, Hinnenbusch 1992, Gasch et al. 2000, Marks et al. 2003).[16]
It is important to note that sulfur dioxide additions do not bind the oxygen and, therefore, do not prevent the first step in this coupled oxidation. Some winemakers use ascorbic acid, or vitamin C, as an antioxidant. Ascorbic acid sometimes protects the fruit and acts as an antioxidant, while at other times it can act as a proto-oxidant, or oxidative promoter.[17]
The two roles of ascorbic acid are mainly the result of concentration and the presence of adequate sulfur dioxide. As illustrated below, when ascorbic acid is added to wine, it binds oxygen rapidly to form two reaction products, dehydroascorbate and hydrogen peroxide. If there is not enough ascorbic acid maintained to react with the oxygen, oxidative degradation, including coupled oxidation, can occur. If there is not adequate sulfur dioxide maintained to bind with the hydrogen peroxide formed by the ascorbic acid, wine oxidation can occur.[17]
Therefore, the keys to optimizing the performance of ascorbic acid as an antioxidant are to maintain a concentration of about 50 mg/L, and to have adequate sulfur dioxide. The use of ascorbic acid involves the following considerations:[17]
- Reaction between ascorbic acid and oxygen much more rapid than SO2
- SO2 does not directly react with oxygen, but mainly with reaction products, such as H2O2
- Optimum levels of ascorbic acid (50 mg/L or more) and more SO2 can prolong the antioxidant phase of ascorbic acid.
- For example: If 100 mg/L ascorbic acid in wine reacts completely with oxygen, 62 mg/L SO2 is required to react with the ascorbic acid oxidation product
The possible use of ascorbic acid should be determined based on the assessment of white wine longevity and oxidation potential. This addition compound may have a place in the production of some delicate, low phenol white wines.
Ascorbic acid has been described to prevent caffeic acid oxidation at pH 7.0 (Cilliers & Singleton, 1990) and to regenerate caffeic acid from phenoxyl radical.[18] The presence of ascorbic acid (1%) either alone or together with EDTA completely prevented caffeic acid degradation during alkaline hydrolysis of bound phenolics with NaOH. the loss of chlorogenic acid during homogenization in the absence of ascorbic acid and EDTA is unlikely to be due to spontaneous autoxidation. More likely, the loss is due to enzymatic oxidation, occurring in fresh fruits by the action of polyphenol oxidase (Coseteng & Lee, 1987; Matheis, 1987). The ability of ascorbic acid to reduce back the phenoxyl radical intermediate produced during oxidation of phenolic compounds is probably responsible for the observed effect. Such a mechanism has been already demonstrated for the phenoxyl radical intermediate derived from caffeic acid oxidation, which is reduced back to caffeic acid by ascorbate
Ascorbic acid added during mashing can reduce lautering time due to its antioxidant effect.[19]
we can demonstrate the very real benefit of ascorbate additions to a mash in terms of protecting against oxidation[20] Cites . Kanauchi, M.; Simon, K.J. and Bamforth, C.W.: Ascorbic acid oxidase in barley and malt and its possible role during mashing. Journal of the American Society of Brewing Chemists, 72 (2014), pp. 30-35.
ascorbic acid used as a beer additive can help compensate for some level of oxidation, but can also catalyze oxidation reactions if the amount is insufficient, making things worse instead of better.[21]
Ascorbic acid is not present in unmalted barley, but it is formed during malting, along with ascorbate peroxidase.[22]
Adding AA had little impact on the specific gravity of the recovered wort. Although the pH of the mash was lowered by AA initially, it rose progressively during mashing. The pH of the control mash decreased. The addition of AA led to markedly higher levels of polyphenol and thiols being measurable in the wort. This result is consistent with reports of AA functioning: AAO consumed oxygen, which was used to oxidize polyphenols and thiols. There is generally also a lower color observed in small‐scale laboratory mashes (with the exception of the 60 min measurement, which was perhaps a spuriously high value). This result is regarded as consistent with less polyphenol oxidation in the mashes in view of the extremely high affinity of AAO II for AA and oxygen, coupled with its thermotolerance. There are some consequences to oxygen ingress in a mash, including possibilities of oxidation of unsaturated fatty acids, cross‐linking of thiol‐rich proteins, and oxidation of polyphenols with the production of color [12]. The addition of AA to mashes is expected to engender diminution in such effects. Bamforth et al. [4] anticipated increased measurable levels of such groups in mashes containing AA. An increased level of polyphenol surviving into wort and a decrease in the amount of color produced would be expected.[22] In short: Addition of ascorbic acid to mashes results in the survival of higher levels of polyphenol and thiols into wort and reduced color in that wort, commensurate with AAO preferentially consuming oxygen. Consequently, in adding ascorbic acid, oxygen is less available for other reactions, such as thiol oxidation and polyphenol oxidation in mashes.
Best known of the nonenzymatic protectants is ascorbic acid, which undergoes the redox interchanges shown in Figure 3. The electrons are lost in oxidation of the ascorbate, providing reducing equivalents that can make ascorbate a powerful antioxidant in food systems. Semidehydroascorbic acid, for the most part, disproportionates (eq. 16) as follows: 2 semidehydroascorbate -> ascorbate +.dehydroascorbate (16). Ascorbate reacts rapidly with superoxide and perhydroxyl and even more rapidly with hydroxyl to yield the semialdehyde. The redox chemistry of ascorbic acid is highly complex. Thus, copperinduced oxidation of ascorbate produces peroxide and hydroxyl and ascorbic-copper mixtures inactivate many enzymes. For example, papain will be inactivated by this couple if the palliatives are introduced into beer simultaneously (28). Ascorbate can reduce Fe(III) to Fe(II) and, in the presence of H2O2, will actually stimulate the formation of hydroxyl (36). Ascorbate is a natural component of barley and malt (71). In various plant tissues (though not yet in barley seeds), the enzyme dehydroascorbate reductase has been shown to restore ascorbate to its reduced form with the intermediacy of glutathione (GSH), the latter being oxidized to its dithiol state (GSSG) (eq. 17).[23]
ASC participates in the initiation of the radical reactions in the beer samples, and so ascorbic acid works as a prooxidant in the degradation of the beer. Consequently, adding ascorbic acid to conventionally-brewed beer may not be desired.[24]
The addition of ascorbate to mashes can preferentially scavenge the oxygen and make for less thiol oxidation, less colour and higher polyphenol levels in the wort.[25]
Addition of sodium ascorbate accelerated the rate of formation of spin adducts, which demonstrates that it acts as a prooxidant during the oxidation of (conventionally-brewed and filtered) beer[26]
Potential sources[edit]
- https://researchoutput.csu.edu.au/ws/portalfiles/portal/9307358/29220
- https://researchoutput.csu.edu.au/ws/portalfiles/portal/8781606/23109manuscript.pdf
- https://onlinelibrary.wiley.com/doi/pdf/10.1002/j.2050-0416.2007.tb00252.x antioxidant effects in beer
- http://phd.lib.uni-corvinus.hu/332/3/nagymate_emese_ten.pdf
- https://www.apps.fst.vt.edu/extension/enology/EN/133.html
- https://www.ncbi.nlm.nih.gov/pubmed/9448835
- "Ascorbic acid oxidase in barley and malt and its possible role during mashing"
- https://pdfs.semanticscholar.org/d497/8ff270e8833966c0d2fa86f3ae61b7827fde.pdf removing chloramine
- http://www.themodernbrewhouse.com/forum/viewtopic.php?p=35369#p35369
- https://themodernbrewhouse.com/forum/viewtopic.php?f=11&t=1885&p=35278
- Ascorbic Acid Oxidase in Barley and Malt and Its Possible Role During Mashing
- Fenton reaction acceleration using maltose and ascorbic acid
- https://www.sciencedirect.com/science/article/abs/pii/S0165022X07001066?via%3Dihub
- https://www.themodernbrewhouse.com/forum/viewtopic.php?f=11&t=2717
- https://www.homebrewtalk.com/threads/vitamin-c-the-game-changer.698328/
- https://www.themodernbrewhouse.com//forum/viewtopic.php?f=11&t=2793
- Baik, B.-K., Czuchajowska, Z., and Pomeranz, Y. 1995. Discoloration of dough for oriental noodles. Cereal Chem. 72:291-296.
- Butttner, G.R., Jurkiewicz, B.A.: Chemistry and biochemistry of ascorbic acid. In: Hanbook of antioxidants, editors Cadenas, E., Packer, L. Marcel Dekker, New York 1996.
- http://www.themodernbrewhouse.com/wp-content/uploads/2017/04/savel_0206.pdf
- Yen G-C, Chen H-Y, Peng H-H. 1997. Antioxidant and pro-oxidant effects of various tea extracts. J Agric Food Chem 45(1):30–4.
References[edit]
- ↑ White, C. https://www.jstrack.org/brewing/Yeast_nutrition_article.pdf
- ↑ https://www.canterbury.ac.nz/media/documents/science-outreach/vitaminc_iodate.pdf
- ↑ Kunze, Wolfgang. Technology Brewing & Malting. Edited by Olaf Hendel, 6th Engligh Edition ed., VBL Berlin, 2019. p. 509.
- ↑ deLange AJ. Campden tablets (sulfites) and brewing water. Homebrew Talk website. 2012. Accessed March 2024.
- ↑ https://www.dudadiesel.com/choose_item.php?id=asc8c
- ↑ https://www.intralabs.co.uk/ascorbic-acid/100g-ascorbic-acid-powder.html
- ↑ Mansfield, AK. "Kicking up a Stink: Treatment for Sulfur Off-Odors." Cellar Dweller. Cornell University. Apr 2010.
- ↑ Irwin, AJ, et al. "The Role of Copper, Oxygen, and Polyphenols in Beer Flavor Instability." Journal of the American Society of Brewing Chemists, vol. 49, no. 3, 1991, pp. 140–149.
- ↑ https://ujcontent.uj.ac.za/vital/access/services/Download/uj:14727/CONTENT1
- ↑ Briggs DE, Boulton CA, Brookes PA, Stevens R. Brewing Science and Practice. Woodhead Publishing Limited and CRC Press LLC; 2004.
- ↑ Quinde-Axtell Z, Powers J. Baik BK. Retardation of discoloration in barley flour gel and dough. Cereal Chem. 2006;83(4):385–390.
- ↑ Chapon L, Chemardin M. The dissolving and oxidation of malt tannoids on mashing-in. Proceedings from the Annual meeting of American Society of Brewing Chemists. 1964;22(1):244–258.
- ↑ Callemien D, Collin S. Structure, organoleptic properties, quantification methods, and stability of phenolic compounds in beer—a review. Food Rev Int. 2009;26(1):1–84.
- ↑ Liégeois C, Lermusieau G, Collin S. Measuring antioxidant efficiency of wort, malt, and hops against the 2,2'-azobis (2-amidinopropane) dihydrochloride-induced oxidation of an aqueous dispersion of linoleic acid. J Agric Food Chem. 2000;48(4):1129–1134.
- ↑ a b c Borsook, H., et al. "The Oxidation of Ascorbic Acid and its Reduction in vitro and in vivo*" J. Biol. Chem. 1937. 117:237-279.
- ↑ Bell, SJ, and Henschke, PA. "Implications of nitrogen nutrition for grapes, fermentation and wine." Australian Journal of Grape and Wine Research. 11, 242–295, 2005.
- ↑ a b c Zoecklein B. Enology Notes #133. Virginia Tech website. 2007. Accessed online March 2024.
- ↑ Nardini M, Cirillo E, Natella F, Mencarelli D, Comisso A, Scaccini C. Detection of bound phenolic acids: prevention by ascorbic acid and ethylenediaminetetraacetic acid of degradation of phenolic acids during alkaline hydrolysis. Food Chem. 2002;79(1):119–124.
- ↑ Karabín M, Hanko V, Nešpor J, Jelínek L, Dostálek P. Hop tannin extract: a promising tool for acceleration of lautering. J Inst Brew. 2018;124(4):374–380.
- ↑ Kanauchi M, Bamforth CW. A Challenge in the study of flavour instability. BrewingScience - Monatsschrift Brauwiss. 2018;71(Sept/Oct):82–84.
- ↑ Nielsen H. The control of oxygen in beer processing. J Inst Brew. 1973;79(2):147–154.
- ↑ a b Kanauchi M. Oxidative enzyme effects in malt for brewing. In: Kanauchi M, ed. Brewing Technology. IntechOpen. 2017:29–47.
- ↑ Bamforth CW, Muller RE, Walker MD. Oxygen and oxygen radicals in malting and brewing: a review. J Am Soc Brew Chem. 1993;51(3):79–88.
- ↑ Brezová V, Polovka M, Staško A. The influence of additives on beer stability investigated by EPR spectroscopy. Spectrochimica Acta Part A. 2002;58(6):1279–1291.
- ↑ https://onlinelibrary.wiley.com/doi/full/10.1002/jib.594
- ↑ Andersen ML, Outtrup H, Skibsted LH. antioxidants in beer assessed by ESR spin trapping. J Agric Food Chem. 2000;48(8):3106–3111.
