Oxidation: Difference between revisions
| Line 131: | Line 131: | ||
== Potential sources == | == Potential sources == | ||
*[http://www.themodernbrewhouse.com/wp-content/uploads/2017/03/Baert-Aldehyden.pdf On the Origin of Free and Bound Staling Aldehydes in Beer] | *[http://www.themodernbrewhouse.com/wp-content/uploads/2017/03/Baert-Aldehyden.pdf On the Origin of Free and Bound Staling Aldehydes in Beer] | ||
*[https://onlinelibrary.wiley.com/doi/pdf/10.1002/jib.644 On the contribution of malt quality and the malting process to the formation of beer staling aldehydes: a review] | *[https://onlinelibrary.wiley.com/doi/pdf/10.1002/jib.644 On the contribution of malt quality and the malting process to the formation of beer staling aldehydes: a review] | ||
*[https://www.mdpi.com/2304-8158/10/10/2320 The Influence of Proteolytic Malt Modification on the Aging Potential of Final Wort] | *[https://www.mdpi.com/2304-8158/10/10/2320 The Influence of Proteolytic Malt Modification on the Aging Potential of Final Wort] | ||
Revision as of 07:10, 3 March 2024


Please check back later for additional changes

Oxidation is the main factor responsible for beer flavor deterioration during the brewing process.[1][2][3][4][5][6][7][8] Oxygen undergoes a complex series of reactions with organic compounds in malt and hops, resulting in the destruction of fresh flavors, and the creation of off-flavors, among other problems.[9][10] This can take place during malting, in the brewhouse, or during aging in the brewery or in the package. Flavor changes are therefore dependent on raw materials, malting and brewing procedures, packaging conditions, and environmental factors.[11] The achievement of enhanced flavor stability of beer demands not only the elimination of oxygen from the finished product but also the avoidance of oxygen ingress throughout the brewing operation, since oxidation of beer components largely occurs as a result of wort processing.[12][13] In particular, mashing requires special attention in order to protect the wort, because the high temperatures, presence of catalysts, and handling of hot wort together with access to oxygen are perfect conditions for oxidation reactions, and the oxidation products created during this stage can influence the stability of the final beer.[14][1][15][16][17][18] Oxidation reactions are generally irreversible (except for some proteins).[10] In other words, once fresh flavors of malt and hops are destroyed, they cannot be regenerated. However oxidation can be slowed or largely eliminated by employing a "low oxygen brewing" method, usually in combination with the use of reducing agents such as sulfites or ascorbic acid.[10] Successful avoidance of oxidation results in a more stable and better-tasting beer, along with health benefits such as higher antioxidant capacity and increased soluble fiber.[7][19][20]
Only a very limited amount of oxidation needs to take place to generate perceptible stale character.[9]
Oxidation will use up the reducing potential in the mash and wort, producing beers with lower reducing potential, which would notionally be more prone to more rapid oxidation.[13]
Effects of oxidation
Oxidation has a profound negative influence on beer flavor:[7][9][13]
- Oxidation causes the destruction of fresh flavors from malt and hops.[21][22][14]
- Oxidation causes the creation of off-flavors, including staleness and unpleasant bitterness and astringency.[23][7][24][25]
Numerous other undesirable effects are seen as well:
- Darker color (resulting from oxidation of phenolic compounds)[21][26][27]
- Slower lautering or circulation speed, and stuck mash is more likely (resulting from oxidation of proteins, arabinoxylans, and other polymers)[21][12]
- Formation of haze (resulting from oxidation of phenolic compounds and proteins)[12][28]
- Decreased foam stability (resulting from oxidation of lipids)[29]
- Decreased antioxidants and antioxidant capacity (resulting from oxidation of antioxidants including phenolic compounds and proteins)[26][12][30]
- Decreased flavor stability (resulting from oxidation of lipids and various antioxidants)[21][26]
- Decreased starch degradation (resulting from oxidation of protein and subsequent gel formation)
- Decreased beta-glucan degradation (resulting from oxidation of endo-β-glucanases)[21][28][26][31]
- Decreased protein degradation and decreased free amino nitrogen (resulting from oxidation of cysteine proteases and oxidative protein aggregation)
- Decreased extract (resulting from oxidative protein aggregation)[17]
- Decreased fermentation performance, i.e. slower fermentation and lower attenuation (resulting from decreased starch degradation, decreased free amino nitrogen, and increased oxidative stress on the yeast)[21][26]
- Decreased body (mouthfeel) in the beer (resulting from oxidation of arabinoxylans)
- Decreased health benefits from consuming the beer (resulting from decreased levels of antioxidants and dietary fiber)
Potential beneficial effects of oxidation:
- Removal of hydrogen sulfide from beer after fermentation (if present)
Factors promoting oxidation
There are three main factors that promote oxidation during the brewing process:[32][33][27][34][28]
- Presence of oxygen (O2)
- Presence of a catalyst (something that speeds up the reaction)
- Heat
Oxygen availability
It should be no surprise that the presence of oxygen is a main factor promoting oxidation since it leads to the generation of reactive oxygen species (more on this below).[35][7] Oxygen gas (O2) that dissovles into liquid water, wort, beer, or other liquid is called dissolved oxygen (DO).
DO can be introduced in the wort during hot-side processing from several sources:
- Water (the main ingredient when mashing) is naturally saturated with DO.[21][9]
- The method of adding grain to water during mashing-in creates air bubbles, which then transfer oxygen to the wort.[21][26]
- Air exposure at the surface of the wort allows oxygen to continually dissolve into the wort, which is increased with circulation, stirring, or any process steps that cause splashing or agitation.[21][26][36][17][9][37][38]
- Circulation through most types of plastic tubing will introduce oxygen because oxygen can diffuse through many plastics. Also, if any fittings are leaky, they can draw in air bubbles.[38]
After the wort is chilled, the wort is intentionally oxygenated:
- Pitching less active yeast may delay the start of fermentation, increasing oxygen exposure.
After fermentation completes, exposure to air/oxygen can easily occur:
- Oxygen can enter the fermentation vessel through airlocks by diffusion, through plastic (especially silicone), through any leaks in the vessel, and at any time the fermenter is opened. Air may also enter the fermenter when beer samples are removed or when the temperature drops.
- Depending on the techniques used, beer may come into contact with air during transfer into the storage vessel (e.g. in a bottling bucket or bright tank).[19] Air may also be present in the storage/serving vessel (e.g. bottles and kegs). Continuing throughout storage, oxygen diffuses through plastics, including beer dispensing lines, gaskets on kegs, and the plastic seal on bottle caps.[10]
- Tanks of CO2 gas used for carbonating and dispensing beer from kegs are not 100% pure carbon dioxide gas; they contain trace amounts of oxygen.[citation needed]
Catalysts
A catalyst increases the speed of a chemical reaction without being consumed by the process. Therefore, catalysts can greatly promote a reaction even in very small amounts.
Malt naturally contains the following enzymes that promote oxidation reactions during mashing (then they are destroyed by boiling):[39][17][2]
- Peroxidases - catalyze the oxidation of phenolic compounds by hydrogen peroxide.[26][12][2][14] These enzymes are highly active throughout mashing and lautering.[28][40][41][17][16][2]
- Polyphenol oxidases - catalyze the oxidation of phenolic compounds by oxygen.[26][42][14]
- Lipoxygenases - catalyze the oxidation of free fatty acids.[35][2]
- Thiol oxidases - catalyze the oxidation of protein thiols. Highly active.[43]
- Ascorbate peroxidase - catalyzes the oxidation of ascorbic acid by hydrogen peroxide (H2O2).[44] Active during mashing, but destroyed quickly.[44]
- Ascorbic acid oxidase - catalyzes the oxidation of ascorbic acid by oxygen (O2).[44] Very active during mashing and fairly heat-stable[43]
- Oxalate oxidase (AKA germin) - catalyses the conversion of oxalate into carbon dioxide and hydrogen peroxide. pH optimum of approximately 4.0 but active over a large range. Because the enzyme is active in a broad pH range, and because it has high heat tolerance, it was active during mashing, but it was less important than other oxidases for scavenging oxygen from mashes because of its low affinity for oxygen.[44] highly active.[43]
- Glucose oxidase?
Transition metals promote oxidation reactions,[45][32][9] and can be introduced from several sources:
- Malt is the primary source of metals.[45][8][46] Depending on the malts used, a standard wort has levels of around 100-270 μg/L iron, 20-400 μg/L copper, and 80-150 μg/L manganese.[45] As little as 10 μg/L of these metals can make a detectable difference to the oxidative stability.[45]
- Hops are a relatively substantial source of manganese in beer (and especially so with dry hopping).[8]
- Brewing liquor may contain metals; for example, some sources of water contain high levels of iron.
- Metallic brewing gear can contribute metals through contact with brewing liquor, wort, or beer; examples include copper wort chillers and stainless steel kegs.[35]
- Brewing additives can contain metals. For example, some yeast nutrient products contain manganese.
Light catalyzes oxidation reactions.[33][28] The influence of light was thought to be overcome by using brown bottles. But even in brown bottles, light exercises an influence during long storage times (for instance on the shelves in supermarkets).[35]
Heat
Higher temperatures increase the speed of oxidation reactions.[42][34][9] Therefore, hot wort (e.g. during mashing) is more susceptible to oxidation than cooled wort and beer (e.g. when pitching yeast).
How oxidation occurs
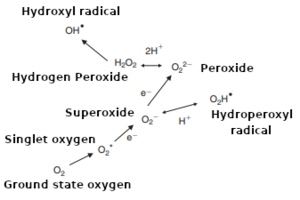
(higher = more reactive)
Groud state oxygen (O2) is relatively stable (unreactive) and needs to be "activated" before developing its damaging impact.[25][12][23][28][45][32][9][8][36] Activation occurs via the partial reduction of O2 to form reactive oxygen species (ROS).[7][16] These ROS react very quickly, damaging many of the organic molecules in wort and beer.[12][7][47][45][38] ROS include superoxide and hydroperoxyl radicals (O2– ⇌ O2H•), hydrogen peroxide (H2O2) and other peroxides (especially linoleic acid hydroperoxide), and the hydroxyl radical (OH•).[2][12][7][16][28] Hydroxyl radicals are the most important intermediates in oxidative reactions.[48][23][32] The term ROS can also refer to nitrogen radicals, or even non-radicals with the potential to oxidize or convert to oxidizing radicals.[25][9] However, ROS are typically formed as a result of metals or other catalysts activating dissolved oxygen (DO) in the wort or beer.[23][32][27][12][49][36] However, this is often just the first step in a chain reaction because when ROS attack organic molecules, the reaction produces additional ROS or other radicals that then go on to damage other organic molecules, and so on.[9][48][23][27] The chain reaction is stopped only when the radicals react with molecules that form stable products when they oxidize, such as sulfites (SO2) or phenolic compounds — these molecules are called antioxidants.[27][9][36][50]
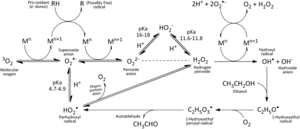
With the exception of zinc, transition metals possess unpaired electrons, and therefore they are radicals.[9] Most notable among these for brewers are iron, copper, and manganese. Through an array of reactions such as Fenton and Haber-Weiss reactions, these metals facilitate electron transfer to catalyze the activation of oxygen to form all the ROS discussed above.[2][32][23][48][45][9][25] Interestingly, some reducing agents such as ascorbic acid and certain phenolic compounds (especially 3',4',5'-trihydroxyflavans) can increase these reaction rates by reducing the metals to their lower redox states, which are primarily responsible for generating oxygen radicals.[32] Transition metals can also catalyze the formation of radicals in beer without the influence of oxygen, such as reacting with unsaturated fatty acids to form lipid radicals.[25][23] Prevention of oxidation must include attention toward limiting and/or removing these catalytic transition metals, instead of solely restricting oxygen.[8]
Activation of oxygen to superoxide by iron:
Fe2+ + O2 → Fe3+ + O2–
Production of ROS is a normal part of biological processes. Barley contains a system of oxygen-scavenging enzymes to help protect itself from the damage occuring from accumulation of superoxide and peroxide.[40][12] The three enzymes in this system are superoxide dismutase, catalase, and peroxidase. Unfortunately for brewers, the system fails during mashing because peroxidase is the only enzyme that survives the high temperature, and by itself it merely catalyzes the oxidation of phenolic compounds by hydrogen peroxide (H2O2).[17] A few other enzymes are notable as well: Polyphenol oxidase may be active during mashing to catalyze oxidation of phenolic compounds. Lipoxygenases catalyze the oxidation of unsaturated fatty acids during mashing (see Lipids). Thiol oxidase catalyzes the oxidation of proteins.[43]
Compounds that oxidize
Oxidation of compounds during the brewing process can be monitored by analysis of redox potential or reducing power.[17]
Lipids
Fatty acids oxidize to form carbonyl compounds through a complex series of reactions.[51][2] Enyzmes such as lipoxygenases, hydroperoxide lyase, and hydroperoxide isomerase can catalyze lipid oxidation, but these enzymes are not necessary since ROS can also directly oxidize lipids.[9][2] Trans-2-nonenal is a notable lipid oxidation product formed during brewing that is released during beer storage and has a papery/cardboard flavor.[32][7] Lipid oxidation produces radicals (e.g. linoleic acid hydroperoxide) that further propogate the oxidation process in wort.[7][48] See Lipids for more discussion.
Phenolic compounds
Phenolic compounds readily oxidize and they are the most abundant substances to absorb ROS in wort and beer. Phenolic compounds can absorb radicals to form relatively stable products, essentially halting the oxidation chain reaction.[9] This makes them valuable protection against oxidation of other compounds. However, the oxidation of phenolic compounds during the brewing process is undesirable since it leads to off-flavors (generally an unpleasant bitterness and astringency), haze formation, darker color, and lower antioxidant capacity of the beer.[35][12][2] Besides ROS-scavenging, phenolics can also help prevent oxidation by chelating transition metals and inhibiting lipoxygenase enzymes.[9] See Phenolic compounds for more information.
Arabinoxylans
Beta-glucanase stability increases if it is separated from peroxidase activity and the powerful anti-oxidant sodium dithionite has the same effect.[28]
Proteins
Oxidation of free thiols (to form disulphide cross-links) can result in the formation of hydrogen peroxide.[28] The generation of hydrogen peroxide as by-product is clearly significant since there is potential for considerable damage to other mash constituents such as flavor compounds.[28] H2O2 can be generated by reaction of thiols with O2, but it is well known that H2O2 will also react with thiols.[12] The reduced form of LTP1 scavenges one of the dominating radical compounds in beer, the 1-hydroxyethyl radical, at a rate similar to other reactive compounds in beer such as hop bitter acids. The native form of LTP1 in barley is stabilized by four disulfide bonds and, if these disulfide bonds are not reduced to free thiols, they are not reactive toward the 1-hydroxyethyl radical.[52] The formation of gel-protein during mashing is, at least partly, related to oxidative enzyme activity during mashing.[40] Most oxygen-scavenging enzymes are able to catalyse the formation of disulphide bridges.[40] the reaction of thiol-containing proteins with oxygen leads to the formation of hydrogen peroxide, H2O2.[12] H2O2 is generated when thiols are oxidized by enzymes (11,15), by copper ions (13), or by other oxidative events occurring within the protein.[17] "A possible working mechanism for the ROS-scavenging ability of LTP1 or other proteins with free thiols: LTP thiol(s) is oxidised to the sulfenic acid by oxidants such as H2O2, which results in the destruction of a peroxide molecule in 1:1 stoichiometry. The free thiol can be recovered by two sequential reactions (reactions 2 and 3). The reaction 2 generates a disulfide (LTP-SSR) through reaction with a small molecule (HS-R) such as yeast thioredoxin. The reaction 3 uses sulfite or phenolic compounds to generate free thiol from the disulfide for the next round elimination of ROS."[7]
- Reaction:[28] R-HS + HS-R' + O2 --> R-S-S-R' + H2O2
iso-α-acids
Hydrogen peroxide accelerates the formation of such staling compounds as aldehydes and the degradation products of the iso-a-acids.[28] Unhopped beers seldom develop an oxidized flavor, which suggests a likely role for the iso- α -acids as precursors of staling compounds ( Hashimoto et al., 1979 ). In model systems it has been shown that volatile carbonyls (alkenals and alkadienals with chain lengths of between 6 and 12 carbon atoms) can be produced from a solution of bitter substances, higher alcohols and melanoidins. The trans isomers are more prone to degradation than are the cis isomers ( De Cooman et al., 2000 ; Araki et al., 2002 ). Reduced side-chain iso- α -acids do not give staling carbonyls ( Hashimoto, 1988 ).[9]
Alcohols
oxygen radicals are very reactive with ethanol, the second most abundant component in beer, leading to the formation of off-flavors and consequent beer deterioration (Andersen and Skibsted 1998; Andersen and others 2000; Vanderhaegen and others 2006).[2] hydroxyl radicals react mainly ethanol to produce 1-hydroxyethyl radicals.[34]
Alcohols in beer can be converted to their equivalent aldehydes through the mediation of melanoidins, with the oxidized carbonyl groups on the latter acting as electron acceptors ( Hashimoto, 1972a ). Devreux et al. (1981) suggest that the reaction is inhibited by polyphenols and requires light, so is of secondary signifi cance in beer. Meanwhile, Irwin et al. (1991) argue that the effi ciency of conversion is so small as to make the pathway irrelevant.[9]
Melanoidins are responsible for the oxidation of higher alcohols to volatile aldehydes, as reported by Hashimoto, leading to beer oxidation and deterioration. The mechanism involves the transference of electrons or hydrogen from alcohols to carbonyl groups of melanoidins in conditions of high temperature and low pH (Hashimoto 1972). The melanoidin-mediated oxidation of higher alcohols, associated with the oxidation of isohumolones and unsatured fatty acids, is responsible for the formation of volatile aldehydes with a negative impact in beer flavor and storage stability (Hashimoto 1977).[2]
Sulfites
The use of radical scavengers could improve beer flavor stability.[23] Sulfite has been identified as an essential antioxidant in beer, which has been ascribed to its ability to remove H2O2.[6]
Hydrogen sulfide
Heterocyclic compounds
Melanoidins
Regarding Maillard reaction products, Andersen et al. (2000) found them to be pro-oxidants, whereas Bright et al. (1999) and Coghe et al. (2003) described the benefit to flavor stability of these materials, especially from more roasted malts.[9] Melanoidins, like phenolic compounds, can absorb radicals to form relatively stable products, essentially halting the oxidation chain reaction.[9]
Potential sources
- On the Origin of Free and Bound Staling Aldehydes in Beer
- On the contribution of malt quality and the malting process to the formation of beer staling aldehydes: a review
- The Influence of Proteolytic Malt Modification on the Aging Potential of Final Wort
- Bamforth. The Horace Brown Medal. Forever in focus: researches in malting and brewing sciences
- Retention of Iron and Copper during Mashing of Roasted Malts
- Estimation of Antioxidative Activity and its Relationship to Beer Flavor Stability
- Anne M. Frederiksen, Rikke M. Festersen and Mogens L. Andersen . Oxidative Reactions during Early Stages of Beer Brewing Studied by Electron Spin Resonance and Spin Trapping. Journal of Agricultural and Food Chemistry 2008, 56 (18) , 8514-8520.
- Emiliano Martinez-Periñan, María P. Hernández-Artiga, José M. Palacios-Santander, Mohammed ElKaoutit, Ignacio Naranjo-Rodriguez, Dolores Bellido-Milla. Estimation of beer stability by sulphur dioxide and polyphenol determination. Evaluation of a Laccase-Sonogel-Carbon biosensor. Food Chemistry 2011, 127 (1) , 234-239.
- Possible implication of four oxidoreductases (polyphonoloxidase, catalase, lipoxygenase, and peroxidase) present in brewery's barley and malt on organoleptic and rheological properties of mash and beer.
- Boivin, P., Clamagirand, V., Maillard, M. N., Berset, C., and Malanda, M. Malt quality and oxidation risk in brewing. Proc. Conv. Inst. Brew. Asia Pacific Sect. 24:110-115, 1996.
- Bamforth, C. W. Oxido-reduction processes and active forms of oxygen in aqueous systems. Cerevisia Belgian J. Brew. Biotechnol. 26:149-154, 2001.
- Bamforth, C. W., Hughes, P. S., Muller, R. E., Walters, M. T., Antrobus, C. J., and Large, P. J. Oxidation in malting and brewing: Assessment, implication and elimination. Proc. Int. Brew. Tech. Conf. 6:267-276, 1996.
- Dadic, M. Beer stability—A key to success in brewing. Tech. Q. Master Brew. Assoc. Am. 21:9-26, 1984.
- Savel, J., Differential spectroscopy and beer oxidation, Techn. Quart. MBAA, 42 (1), 57 – 64, 2005.
- Chapon, L., Chapon, S.: Peroxidatic step in oxidation of beer, J. Am. Soc. Brew. Chem., 37, 96 – 104, 1979.
- Cortés, K., Suárez, H., & Methner. (2010). Development and correlation between the organic radical concentration in different malt types and oxidative beer stability. Journal of the American Society of Brewing Chemists, 68, 107–113.
- Narziss, L., Reicheneder, E., and St. Lustig, W. Oxygen optimization in wort preparation: New findings in pilot-plant and commercialscale tests. Brauweltlnt. 38:238, 1989.
- Lerch, K. Tyrosinase: Molecular and active-site structure. In Enzymatic Browning and Its PreVention; Lee, C., Whitaker, J., Eds.; ACS Symposium Series 600; American Chemical Society: Washington, DC, 1995; pp 64-80.
- Zawistowski, J.; Biliaderis, C. G.; Eskin, N. A. M. Polyphenol oxidase. In OxidatiVe Enzymes in Foods; Robinson, D., Eskin, N., Eds.; Elsevier Science: London, New York, 1991; pp 217- 273.
- https://www.applerubber.com/hot-topics-for-engineers/the-permeability-of-rubber-compounds/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7916471/
- http://www.themodernbrewhouse.com/brewing-methods/cold-fermentation-and-spunding-results/
- http://www.themodernbrewhouse.com/uncategorized/what-does-oxidation-look-like/
- http://www.milkthefunk.com/wiki/Aging_and_Storage#General_Effects_of_Oxidation
- Andersen, M. L., Outtrup, H., and Skibsted, L. H. Potential antioxidants in beer assessed by ESR spin trapping. J. Agric. Food Chem. 48: 3106-3111, 2000.
- Uchida, M., and Ono, M. Determination of hydrogen peroxide and its role in beer oxidation. J. Am. Soc. Brew. Chem. 57:145-150, 1999.
References
- ↑ a b Mertens T, Kunz T, Gibson, BR. Transition metals in brewing and their role in wort and beer oxidative stability: a review. J Inst Brew. 2022;128(3):77–95.
- ↑ a b c d e f g h i j k l Carvalho DO, Gonçalves LM, Guido LF. Overall antioxidant properties of malt and how they are influenced by the individual constituents of barley and the malting process. Compr Rev Food Sci Food Saf. 2016;15(5):927–943.
- ↑ Zhao H, Chen W, Lu J, Zhao M. Phenolic profiles and antioxidant activities of commercial beers. Food Chem. 2010;119(3):1150–1158.
- ↑ Dvořáková M, Douanier M, Jurková M, Kellner V, Dostálek P. Comparison of antioxidant activity of barley (Hordeum vulgare L.) and malt extracts with the content of free phenolic compounds measured by high performance liquid chromatography coupled with CoulArray detector. J Inst Brew. 2008;114(2):150–159.
- ↑ Prechtl C. Some practical observations concerning grain bitterness in beers and its amelioration. Tech Q Master Brew Assoc Am. 1967;4(1):98–103.
- ↑ a b Lund MN, Andersen ML. Detection of Thiol Groups in Beer and Their Correlation with Oxidative Stability. J Am Soc Brew Chem. 2011;69(3):163–169.
- ↑ a b c d e f g h i j k Wu MJ, Clarke FM, Rogers PJ, et al. Identification of a protein with antioxidant activity that is important for the protection against beer ageing. Int J Mol Sci. 2011;12(9):6089–6103.
- ↑ a b c d e Mertens T, Kunz T, Methner FJ. Assessment of chelators in wort and beer model solutions. BrewingScience. 2020;73(May/June):58–67.
- ↑ a b c d e f g h i j k l m n o p q r s t u Bamforth CW, Lentini A. The flavor instability of beer. In: Bamforth CW, ed. Beer: A Quality Perspective. Academic Press; 2009:85–109.
- ↑ a b c d Savel J. Negative role of oxidised polyphenols and reductones in beer. BrewingScience - Monatsschrift Brauwiss. 2006;59(1/2):33–40.
- ↑ Guido LF, Curto AF, Boivin P, Benismail N, Gonçalves CR, Barros AA. Correlation of malt quality parameters and beer flavor stability: multivariate analysis. J Agric Food Chem. 2007;55(3):728–733.
- ↑ a b c d e f g h i j k l m Stephenson WH, Biawa JP, Miracle RE, Bamforth CW. Laboratory-scale studies of the impact of oxygen on mashing. J Inst Brew. 2003;109(3):273–283.
- ↑ a b c O'Rourke T. The role of oxygen in brewing. Brewer International. 2002;2(3):45–47.
- ↑ a b c d Lewis MJ, Bamforth CW. Chapter 12: Oxygen. In: Lewis MJ, Bamforth CW, eds. Essays in Brewing Science. Springer; 2006:131–142.
- ↑ Andersen ML, Skibsted LH. Modification of the levels of polyphenols in wort and beer by addition of hexamethylenetetramine or sulfite during mashing. J Agric Food Chem. 2001;49(11):5232–5237.
- ↑ a b c d Clarkson SP, Large SJ, Bamforth CW. Oxygen-scavenging enzymes in barley and malt and their effects during mashing. J Inst Brew. 1992;98(2):111–115
- ↑ a b c d e f g h Muller R. Use of 5,5’-dithiobis (2-nitrobenzoic acid) as a measure of oxidation during mashing. J Am Soc Brew Chem. 1995;53(2):53–58.
- ↑ Chapon L, Chemardin M. The dissolving and oxidation of malt tannoids on mashing-in. Proceedings from the Annual meeting of American Society of Brewing Chemists. 1964;22(1):244–258.
- ↑ a b Zhao H, Zhao M. Effects of mashing on total phenolic contents and antioxidant activities of malts and worts. Int J Food Sci Technol. 2012;47(2):240-247.
- ↑ Briggs DE, Boulton CA, Brookes PA, Stevens R. Brewing Science and Practice. Woodhead Publishing Limited and CRC Press LLC; 2004.
- ↑ a b c d e f g h i Kunze W. Hendel O, ed. Technology Brewing & Malting. 6th ed. VLB Berlin; 2019:250.
- ↑ Fix G. Principles of Brewing Science. 2nd ed. Brewers Publications; 1999.
- ↑ a b c d e f g h Zufall C, Tyrell Th. The influence of heavy metal ions on beer flavour stability. J Inst Brew. 2008;114(2):134–142.
- ↑ Guido LF, Boivin P, Benismail N, Gonçalves CR, Barros AA. An early development of the nonenal potential in the malting process. Eur Food Res Technol. 2005;220:200–206.
- ↑ a b c d e f Aron PM, Shellhammer TH. A discussion of polyphenols in beer physical and flavour stability. J Inst Brew. 2010;116(4):369–380.
- ↑ a b c d e f g h i Narziss L, Back W, Gastl M, Zarnkow M. Abriss der Bierbrauerei. 8th ed. Weinheim, Germany: Wiley-VCH Verlag GmbH & Co. KGaA; 2017.
- ↑ a b c d e Kunz T, Brandt NO, Seewald T, Methner FJ. Carbohydrates addition during brewing – effects on oxidative processes and formation of specific ageing compounds. BrewingScience. 2015;68(7):78–92.
- ↑ a b c d e f g h i j k l Muller R. The formation of hydrogen peroxide during oxidation of thiol-containing proteins. J Inst Brew. 1997;103(5):307–310.
- ↑ Evans E. Mashing. American Society of Brewing Chemists and Master Brewers Association of the Americas; 2021.
- ↑ Arts MJTJ, Grun C, De Jong RL, et al. Oxidative degradation of lipids during mashing. J Agric Food Chem. 2007;55(17):7010–7014.
- ↑ Jin YL, Speers RA, Paulson AT, Stewart RJ. Barley β-glucans and their degradation during malting and brewing. Tech Q Master Brew Assoc Am. 2004;41(3):231–240.
- ↑ a b c d e f g h Irwin AJ, Barker RL, Pipasts P. The role of copper, oxygen, and polyphenols in beer flavor instability. J Am Soc Brew Chem. 1991;49(3):140–149.
- ↑ a b Hawkins CL, Morgan PE, Davies MJ. Quantification of protein modification by oxidants. Free Radic Biol Med. 2009;46(8):965–988.
- ↑ a b c Callemien D, Collin S. Structure, organoleptic properties, quantification methods, and stability of phenolic compounds in beer—a review. Food Rev Int. 2009;26(1):1–84.
- ↑ a b c d e Narziss L. Technological factors of flavour stability. J Inst Brew. 1986;92:346–353.
- ↑ a b c d Bamforth CW, Muller RE, Walker MD. Oxygen and oxygen radicals in malting and brewing: a review. J Am Soc Brew Chem. 1993;51(3):79–88.
- ↑ Bamforth CW. Enzymic and non‐enzymic oxidation in the brewhouse: A theoretical consideration. J Inst Brew. 1999;105(4):237–242.
- ↑ a b c Nielsen H. The control of oxygen in beer processing. J Inst Brew. 1973;79(2):147–154.
- ↑ Kunze W. Hendel O, ed. Technology Brewing & Malting. 6th ed. VLB Berlin; 2019:214.
- ↑ a b c d Pöyri S, Mikola M, Sontag-Strohm T, Kaukovirta-Norja A, Home S. The formation and hydrolysis of barley malt gel-protein under different mashing conditions. J Inst Brew. 2002;108(2):261–267.
- ↑ Wu MJ, Rogers PJ, Clarke FM. 125th anniversary review: The role of proteins in beer redox stability. J Inst Brew. 2012;118(1):1–11.
- ↑ a b Quinde-Axtell Z, Powers J. Baik BK. Retardation of discoloration in barley flour gel and dough. Cereal Chem. 2006;83(4):385–390.
- ↑ a b c d Kanauchi M, Bamforth CW. A Challenge in the study of flavour instability. BrewingScience - Monatsschrift Brauwiss. 2018;71(Sept/Oct):82–84.
- ↑ a b c d Kanauchi M. Oxidative enzyme effects in malt for brewing. In: Kanauchi M, ed. Brewing Technology. IntechOpen. 2017:29–47.
- ↑ a b c d e f g h Mertens T, Kunz T, Wietstock PC, Methner FJ. Complexation of transition metals by chelators added during mashing and impact on beer stability. J Inst Brew. 2021;127(4):345–357.
- ↑ Maia CR. Stability of beer through control of minerals in sweet wort. Master's thesis. University of Porto. 2018.
- ↑ Gao Y, Fang L, Wang X, et al. Antioxidant activity evaluation of dietary flavonoid hyperoside using Saccharomyces cerevisiae as a model. Molecules. 2019;24(4):788.
- ↑ a b c d Martinez-Gomez A, Caballero I, Blanco CA. Phenols and melanoidins as natural antioxidants in beer. Structure, reactivity and antioxidant activity. Biomolecules. 2020;10(3):400.
- ↑ Wannenmacher J, Gastl M, Becker T. Phenolic substances in beer: Structural diversity, reactive potential and relevance for brewing process and beer quality. Compr Rev Food Sci Food Saf. 2018;17(4):953–988.
- ↑ Liégeois C, Lermusieau G, Collin S. Measuring antioxidant efficiency of wort, malt, and hops against the 2,2'-azobis (2-amidinopropane) dihydrochloride-induced oxidation of an aqueous dispersion of linoleic acid. J Agric Food Chem. 2000;48(4):1129–1134.
- ↑ Kunze W. Hendel O, ed. Technology Brewing & Malting. 6th ed. VLB Berlin; 2019:250:231–232.
- ↑ Lund MN, Petersen MA, Andersen ML, Lunde C. Effect of protease treatment during mashing on protein-derived thiol content and flavor stability of beer during storage. J Am Soc Brew Chem. 2015;73(3):287–295.